Comparison of Mesenchymal Stem Cell Efficacy in Ischemic Versus Nonischemic Dilated Cardiomyopathy
- PMID: 30005555
- PMCID: PMC6064862
- DOI: 10.1161/JAHA.117.008460
Comparison of Mesenchymal Stem Cell Efficacy in Ischemic Versus Nonischemic Dilated Cardiomyopathy
Abstract
Background: Ischemic cardiomyopathy (ICM) and dilated cardiomyopathy (DCM) differ in histopathology and prognosis. Although transendocardial delivery of mesenchymal stem cells is safe and provides cardiovascular benefits in both, a comparison of mesenchymal stem cell efficacy in ICM versus DCM has not been done.
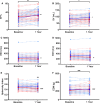
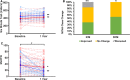
Methods and results: We conducted a subanalysis of 3 single-center, randomized, and blinded clinical trials: (1) TAC-HFT (Transendocardial Autologous Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells in Ischemic Heart Failure Trial); (2) POSEIDON (A Phase I/II, Randomized Pilot Study of the Comparative Safety and Efficacy of Transendocardial Injection of Autologous Mesenchymal Stem Cells Versus Allogeneic Mesenchymal Stem Cells in Patients With Chronic Ischemic Left Ventricular Dysfunction Secondary to Myocardial Infarction); and (3) POSEIDON-DCM (Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy). Baseline and 1-year cardiac structure and function and quality-of-life data were compared in a post hoc pooled analysis including ICM (n=46) and DCM (n=33) patients who received autologous or allogeneic mesenchymal stem cells. Ejection fraction improved in DCM by 7% (within-group, P=0.002) compared to ICM (1.5%; within-group, P=0.14; between-group, P=0.003). Similarly, stroke volume increased in DCM by 10.59 mL (P=0.046) versus ICM (-0.2 mL; P=0.73; between-group, P=0.02). End-diastolic volume improved only in ICM (10.6 mL; P=0.04) and end-systolic volume improved only in DCM (17.8 mL; P=0.049). The sphericity index decreased only in ICM (-0.04; P=0.0002). End-diastolic mass increased in ICM (23.1 g; P<0.0001) versus DCM (-4.1 g; P=0.34; between-group, P=0.007). The 6-minute walk test improved in DCM (31.1 m; P=0.009) and ICM (36.3 m; P=0.006) with no between-group difference (P=0.79). The New York Heart Association class improved in DCM (P=0.005) and ICM (P=0.02; between-group P=0.20). The Minnesota Living with Heart Failure Questionnaire improved in DCM (-19.5; P=0.002) and ICM (-6.4; P=0.03; δ between-group difference P=0.042) patients.
Conclusions: Mesenchymal stem cell therapy is beneficial in DCM and ICM patients, despite variable effects on cardiac phenotypic outcomes. Whereas cardiac function improved preferentially in DCM patients, ICM patients experienced reverse remodeling. Mesenchymal stem cell therapy enhanced quality of life and functional capacity in both etiologies.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifiers: TAC-HFT: NCT00768066, POSEIDON: NCT01087996, POSEIDON-DCM: NCT01392625.
Keywords: functional capacity impairment; mesenchymal stem cell; remodeling heart failure; stem cell.
© 2018 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures
References
-
- Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El‐Khorazaty J, Khan A, Mushtaq M, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Alfonso CE, Valasaki K, Pujol MV, Golpanian S, Ghersin E, Fishman JE, Pattany P, Gomes SA, Delgado C, Miki R, Abuzeid F, Vidro‐Casiano M, Premer C, Medina A, Porras V, Hatzistergos KE, Anderson E, Mendizabal A, Mitrani R, Heldman AW. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON‐DCM trial. J Am Coll Cardiol. 2017;69:526–537. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

