The prevention of 2,4-dinitrochlorobenzene-induced inflammation in atopic dermatitis-like skin lesions in BALB/c mice by Jawoongo
- PMID: 30005655
- PMCID: PMC6045835
- DOI: 10.1186/s12906-018-2280-z
The prevention of 2,4-dinitrochlorobenzene-induced inflammation in atopic dermatitis-like skin lesions in BALB/c mice by Jawoongo
Abstract
Background: Jawoongo is an herbal mixture used in traditional medicine to treat skin diseases. This study aimed to investigate whether Jawoongo ameliorates Atopic dermatitis (AD)-like pathology in mice and to understand its underlying cellular mechanisms.
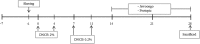
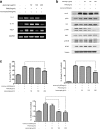
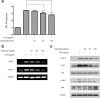
Methods: AD was induced by 2, 4-Dinitrocholrlbenzene (DNCB) in BALB/c mice. Treatment with Jawoongo was assessed to study the effect of Jawoongo on AD in mice. Histological Analysis, blood analysis, RT-PCR, western blot analysis, ELISA assay and cell viability assay were performed to verify the inhibitory effect of Jawoongo on AD in mice.
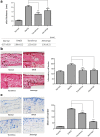
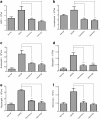
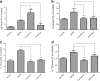
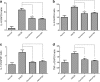
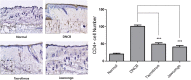
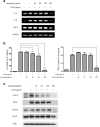
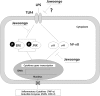
Results: We found that application of Jawoongo in an ointment form on AD-like skin lesions on DNCB-exposed BALB/c mice reduced skin thickness and ameliorated skin infiltration with inflammatory cells, mast cells and CD4+ cells. The ointment also reduced the mRNA levels of IL-2, IL-4, IL-13 and TNF-α in the sensitized skin. Leukocyte counts and the levels of IgE, IL-6, IL-10 and IL-12 were decreased in the blood of the DNCB-treated mice. Furthermore, studies on cultured cells demonstrated that Jawoongo exhibits anti-inflammatory activities, including the suppression of proinflammatory cytokine expression, nitric oxide (NO) production, and inflammation-associated molecule levels in numerous types of agonist-stimulated innate immune cell, including human mast cells (HMC-1), murine macrophage RAW264.7 cells, and splenocytes isolated from mice.
Conclusion: These findings indicate that Jawoongo alleviates DNCB-induced AD-like symptoms via the modulation of several inflammatory responses, indicating that Jawoongo might be a useful drug for the treatment of AD.
Keywords: 2,4-dinitrochlorobenzene; Atopic dermatitis; Cytokine; Inflammation; Jawoongo.
Conflict of interest statement
Ethics approval and consent to participate
Animal experiments were approved by Kyung Hee university institutional animal care of use committee (Approval No. KHUASP(SE)-12–014).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Effects of Angelicae dahuricae Radix on 2, 4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in mice model.BMC Complement Altern Med. 2017 Feb 7;17(1):98. doi: 10.1186/s12906-017-1584-8. BMC Complement Altern Med. 2017. PMID: 28173791 Free PMC article.
-
Inhibitory effect of 5,6-dihydroergosteol-glucoside on atopic dermatitis-like skin lesions via suppression of NF-κB and STAT activation.J Dermatol Sci. 2015 Sep;79(3):252-61. doi: 10.1016/j.jdermsci.2015.06.005. Epub 2015 Jun 15. J Dermatol Sci. 2015. PMID: 26100037
-
Cynanchum atratum inhibits the development of atopic dermatitis in 2,4-dinitrochlorobenzene-induced mice.Biomed Pharmacother. 2017 Jun;90:321-327. doi: 10.1016/j.biopha.2017.03.065. Epub 2017 Mar 30. Biomed Pharmacother. 2017. PMID: 28365521
-
FGF-21 fusion proteins ameliorate atopic dermatitis by inhibiting the TLR/TSLP signaling pathway: Anti-inflammatory and skin barrier repair effects.Int Immunopharmacol. 2025 Jun 26;159:114920. doi: 10.1016/j.intimp.2025.114920. Epub 2025 May 26. Int Immunopharmacol. 2025. PMID: 40424659 Review.
-
Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis.Allergy. 2020 Jan;75(1):54-62. doi: 10.1111/all.13954. Epub 2019 Jul 15. Allergy. 2020. PMID: 31230370 Review.
Cited by
-
Salvianolic Acid A Suppresses DNCB-Induced Atopic Dermatitis-Like Symptoms in BALB/c Mice.Evid Based Complement Alternat Med. 2021 Oct 14;2021:7902592. doi: 10.1155/2021/7902592. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34691223 Free PMC article.
-
Gardenia Jasminoides Ameliorates Antibiotic-Associated Aggravation of DNCB-Induced Atopic Dermatitis by Restoring the Intestinal Microbiome Profile.Nutrients. 2021 Apr 18;13(4):1349. doi: 10.3390/nu13041349. Nutrients. 2021. PMID: 33919521 Free PMC article.
-
Pharmacological Effects of Shikonin and Its Potential in Skin Repair: A Review.Molecules. 2023 Dec 5;28(24):7950. doi: 10.3390/molecules28247950. Molecules. 2023. PMID: 38138440 Free PMC article. Review.
-
Erigeron annuus Extract Improves DNCB-Induced Atopic Dermatitis in a Mouse Model via the Nrf2/HO-1 Pathway.Nutrients. 2024 Feb 3;16(3):451. doi: 10.3390/nu16030451. Nutrients. 2024. PMID: 38337735 Free PMC article.
-
Therapeutic effect of bosentan on 2, 4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis mouse model.Arch Dermatol Res. 2025 Feb 18;317(1):436. doi: 10.1007/s00403-025-03955-z. Arch Dermatol Res. 2025. PMID: 39966154
References
-
- Shirinbak S, Taher YA, Maazi H, Gras R, van Esch BC, Henricks PA, Samsom JN, Verbeek JS, Lambrecht BN, van Oosterhout AJ, et al. Suppression of Th2-driven airway inflammation by allergen immunotherapy is independent of B cell and Ig responses in mice. J Immunol. 2010;185(7):3857–3865. doi: 10.4049/jimmunol.0903909. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

