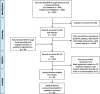
Development of rapid guidelines: 1. Systematic survey of current practices and methods
- PMID: 30005712
- PMCID: PMC6044042
- DOI: 10.1186/s12961-018-0327-8
Development of rapid guidelines: 1. Systematic survey of current practices and methods
Abstract
Background: Guidelines in the healthcare field generally should contain evidence-based recommendations to inform healthcare decisions. Guidelines often require 2 years or more to develop, but certain circumstances necessitate the development of rapid guidelines (RGs) in a short period of time. Upholding methodological rigor while meeting the reduced development timeframe presents a challenge for developing RGs. Our objective was to review current practices and standards for the development of RGs. This is the first of a series of three articles addressing methodological issues around RGs.
Methods: We conducted a systematic survey of methods manuals and published RGs to identify reasons for the development of RGs. Data sources included existing guideline manuals, published RGs, Trip Medical Database, MEDLINE, EMBASE and communication with guideline developers until February 2018.
Results: We identified 46 guidelines that used a shortened timeframe for their development. Nomenclature describing RGs varied across organisations, wherein the United States Centers for Disease Control and Prevention produced 'Interim Guidelines', the National Institute for Health and Care Excellence in the United Kingdom developed 'Short Clinical Guidelines', and WHO provided 'Rapid Advice'. The rationale for RGs included response to emergencies, rapid increases in cases of a condition or disease severity, or new evidence regarding treatment. In general, the methods to assess the quality of evidence, the consensus process and the management of the conflict of interest were not always clear. While we identified another 11 RGs from other institutions, there was no reference to timeframe and reasons for conducting a RG. The three organisations mentioned above provide guidance for the development of RGs.
Conclusions: There is a lack of standardised nomenclature and definitions regarding RGs and there is inconsistency in the methods described in manuals and in RG. It is therefore important that all RGs provide a detailed and transparent description of their methods in order for readers and end-users to be able to assess their quality and validate their findings.
Keywords: Clinical guidelines; Emergencies; Guideline; Guideline development; Methodology; Rapid reviews.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- World Health Organization . WHO Handbook for Guideline Development. Geneva: World Health Organization; 2014.
-
- Schünemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, Ventresca M, Brignardello-Petersen R, Laisaar K-T, Kowalski S. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. Can Med Assoc J. 2014;186(3):E123–E142. doi: 10.1503/cmaj.131237. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources