Host-Derived Serine Protease Inhibitor 6 Provides Granzyme B-Independent Protection of Intestinal Epithelial Cells in Murine Graft-versus-Host Disease
- PMID: 30006303
- PMCID: PMC6286216
- DOI: 10.1016/j.bbmt.2018.07.003
Host-Derived Serine Protease Inhibitor 6 Provides Granzyme B-Independent Protection of Intestinal Epithelial Cells in Murine Graft-versus-Host Disease
Abstract
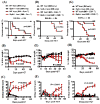
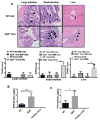
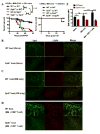
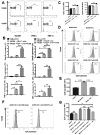
Graft-versus-host disease (GVHD) is a serious complication after allogeneic hematopoietic cell transplantation (allo-HCT) that limits the therapeutic potential of this treatment. Host antigen-presenting cells (APCs) play a vital role in activating donor T cells that subsequently use granzyme B (GzmB) and other cytotoxic molecules to damage host normal tissues. Serine protease inhibitor 6 (Spi6), known as the sole endogenous inhibitor of GzmB, has been implicated in protecting T cells and APCs against GzmB-inflicted damage. In this study we used murine models to examine the previously unknown role of host-derived Spi6 in GVHD pathogenesis. Our results indicated that host Spi6 deficiency exacerbated GVHD as evidenced by significantly increased lethality and clinical and histopathologic scores. Using bone marrow chimera system, we found that Spi6 in nonhematopoietic tissue played a dominant role in protecting against GVHD and was significantly upregulated in intestinal epithelial cells after allo-HCT, whereas Spi6 in hematopoietic APCs surprisingly suppressed alloreactive T cell response. Interestingly, the protective effect of Spi6 and its expression in intestinal epithelial cells appeared to be independent of donor-derived GzmB. We used in silico modeling to explore potential targets of Spi6. Interaction tested in silico demonstrated that Spi6 could inhibit caspase-3 and caspase-8 with the same functional loop that inhibits GzmB but was not capable of forming stable interaction with caspase-1 or granzyme A. Using an in vitro co-culture system, we further identified that donor T cell-derived IFN-γ was important for inducing Spi6 expression in an intestinal epithelial cell line. Altogether, our data indicate that host Spi6 plays a novel, GzmB-independent role in regulating alloreactive T cell response and protecting intestinal epithelial cells. Therefore, enhancing host-derived Spi6 function has the potential to reduce GVHD.
Keywords: Allogenic transplantation; GVHD; Granzyme B; Spi6.
Copyright © 2018. Published by Elsevier Inc.
Conflict of interest statement
The authors have no special/competing interests to disclose.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

