Hide or defend, the two strategies of lymphoma immune evasion: potential implications for immunotherapy
- PMID: 30006449
- PMCID: PMC6068015
- DOI: 10.3324/haematol.2017.184192
Hide or defend, the two strategies of lymphoma immune evasion: potential implications for immunotherapy
Abstract
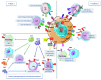
Evading immune eradication is a prerequisite for neoplastic progression and one of the hallmarks of cancer. Here, we review the different immune escape strategies of lymphoma and classify them into two main mechanisms. First, lymphoma cells may "hide" to become invisible to the immune system. This can be achieved by losing or downregulating MHC and/or molecules involved in antigen presentation (including antigen processing machinery and adhesion molecules), thereby preventing their recognition by the immune system. Second, lymphoma cells may "defend" themselves to become resistant to immune eradication. This can be achieved in several ways: by becoming resistant to apoptosis, by expressing inhibitory ligands that deactivate immune cells and/or by inducing an immunosuppressive (humoral and cellular) microenvironment. These immune escape mechanisms may have therapeutic implications. Their identification may be used to guide "personalized immunotherapy" for lymphoma.
Copyright© 2018 Ferrata Storti Foundation.
Figures
References
-
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144(5):646–674. - PubMed
-
- Chen DS, Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity. 2013;39(1):1–10. - PubMed
-
- Goodnow CC, Sprent J, de St Groth BF, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435(7042):590–597. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

