Zika convalescent macaques display delayed induction of anamnestic cross-neutralizing antibody responses after dengue infection
- PMID: 30006514
- PMCID: PMC6045599
- DOI: 10.1038/s41426-018-0132-z
Zika convalescent macaques display delayed induction of anamnestic cross-neutralizing antibody responses after dengue infection
Abstract
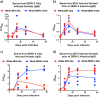
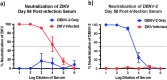
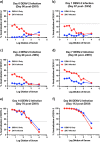
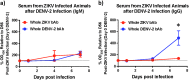
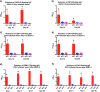
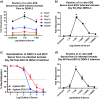
Structural similarities between Zika (ZIKV) and dengue virus (DENV) leads to the induction of cross-reactive responses. We have previously demonstrated that ZIKV exposed macaques significantly enhance DENV viremia. Here we show that this enhancement of DENV infection occurred in the presence of high levels of DENV cross-reactive IgG1 subclass of binding antibodies (bAb) with low DENV neutralizing antibody (nAb) activity (<1:10). The DENV-2 nAb titres after ZIKV infection were, however, higher than those induced in DENV-2 only infected animals suggesting that ZIKV induced low titres of cross-nAb against DENV. Surprisingly, DENV-2 infection of animals previously infected with ZIKV was not accompanied by an anamnestic increase in cross-nAb titres till about 1 week after DENV-2 infection. This delay coincided with enhanced DENV-2 viremia indicating that high levels of cross-bAb in the absence of high nAb contributes to enhancement of DENV infection. Serum collected 8 weeks after DENV-2 infection had high levels of nAb and showed delayed antibody dependent enhancement (ADE) of infection (1:100 dilution) as compared with serum that was collected from ZIKV infected animals prior to DENV-2 infection (1:10 dilution). Examination of serum from macaques that were simultaneously infected with both ZIKV and DENV-2 showed high levels of nAb and delayed ADE responses raising the possibility that the low levels of cross-nAb induced by ZIKV infection could be overcome by co-immunization against ZIKV and DENV infection. Taken together, our results provide additional insights into the nature and kinetics of cross-reactive antibody responses and identify a critical correlate that could potentially prevent enhancement of DENV infection during ZIKV convalescence.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
