Neutralization of the Plasmodium-encoded MIF ortholog confers protective immunity against malaria infection
- PMID: 30006528
- PMCID: PMC6045615
- DOI: 10.1038/s41467-018-05041-7
Neutralization of the Plasmodium-encoded MIF ortholog confers protective immunity against malaria infection
Abstract
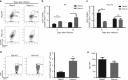
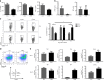
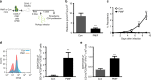
Plasmodium species produce an ortholog of the cytokine macrophage migration inhibitory factor, PMIF, which modulates the host inflammatory response to malaria. Using a novel RNA replicon-based vaccine, we show the impact of PMIF immunoneutralization on the host response and observed improved control of liver and blood-stage Plasmodium infection, and complete protection from re-infection. Vaccination against PMIF delayed blood-stage patency after sporozoite infection, reduced the expression of the Th1-associated inflammatory markers TNF-α, IL-12, and IFN-γ during blood-stage infection, augmented Tfh cell and germinal center responses, increased anti-Plasmodium antibody titers, and enhanced the differentiation of antigen-experienced memory CD4 T cells and liver-resident CD8 T cells. Protection from re-infection was recapitulated by the adoptive transfer of CD8 or CD4 T cells from PMIF RNA immunized hosts. Parasite MIF inhibition may be a useful approach to promote immunity to Plasmodium and potentially other parasite genera that produce MIF orthologous proteins.
Conflict of interest statement
Yale University and Novartis AG have filed a joint patent application describing the potential utility of a
Figures





Comment in
-
Novel malaria vaccines.Hum Vaccin Immunother. 2021 Nov 2;17(11):4549-4552. doi: 10.1080/21645515.2021.1947762. Epub 2021 Aug 4. Hum Vaccin Immunother. 2021. PMID: 34347570 Free PMC article.
References
-
- World Health Organization. World Malaria Report 2014 (World Health Organization, 2014).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

