Inflammation-linked adaptations in dermal microvascular reactivity accompany the development of obesity and type 2 diabetes
- PMID: 30006585
- PMCID: PMC6223541
- DOI: 10.1038/s41366-018-0148-4
Inflammation-linked adaptations in dermal microvascular reactivity accompany the development of obesity and type 2 diabetes
Abstract
Background/objectives: The increased prevalence of obesity has prompted great strides in our understanding of specific adipose depots and their involvement in cardio-metabolic health. However, the impact of obesity on dermal white adipose tissue (dWAT) and dermal microvascular functionality remains unclear. This study aimed to investigate the temporal changes that occur in dWAT and dermal microvascular functionality during the development of diet-induced obesity and type 2 diabetes in mice.
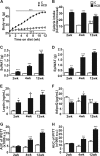
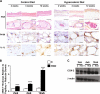
Methods: Metabolic phenotyping of a murine model of hypercaloric diet (HCD)-induced obesity and type 2 diabetes was performed at three time points that reflected three distinct stages of disease development; 2 weeks of HCD-overweight-metabolically healthy, 4 weeks of HCD-obese-prediabetic and 12 weeks of HCD-obese-type 2 diabetic mice. Expansion of dWAT was characterized histologically, and changes in dermal microvascular reactivity were assessed in response to pressure and the vasodilators SNP and Ach.
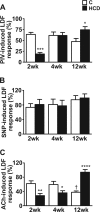
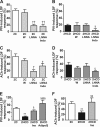
Results: HCD resulted in a progressive expansion of dWAT and increased expression of pro-inflammatory markers (IL1β and COX-2). Impairments in pressure-induced (PIV) and Ach-induced (endothelium-dependent) vasodilation occurred early, in overweight-metabolically healthy mice. Residual vasodilatory responses were NOS-independent but sensitive to COX inhibition. These changes were associated with reductions in NO and adiponectin bioavailability, and rescued by exogenous adiponectin or hyperinsulinemia. Obese-prediabetic mice continued to exhibit impaired Ach-dependent vasodilation but PIV appeared normalized. This normalization coincided with elevated endogenous adiponectin and insulin levels, and was sensitive to NOS, COX and PI3K, inhibition. In obese-type 2 diabetic mice, both Ach-stimulated and pressure-induced vasodilatory responses were increased through enhanced COX-2-dependent prostaglandin response.
Conclusions: We demonstrate that the development of obesity, metabolic dysfunction and type 2 diabetes, in HCD-fed mice, is accompanied by increased dermal adiposity and associated metaflammation in dWAT. Importantly, these temporal changes are also linked to disease stage-specific dermal microvascular reactivity, which may reflect adaptive mechanisms driven by metaflammation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Dermal white adipose tissue undergoes major morphological changes during the spontaneous and induced murine hair follicle cycling: a reappraisal.Arch Dermatol Res. 2018 Jul;310(5):453-462. doi: 10.1007/s00403-018-1831-y. Epub 2018 Apr 27. Arch Dermatol Res. 2018. PMID: 29704126
-
The roles of dermal white adipose tissue loss in scleroderma skin fibrosis.Curr Opin Rheumatol. 2017 Nov;29(6):585-590. doi: 10.1097/BOR.0000000000000437. Curr Opin Rheumatol. 2017. PMID: 28800024 Review.
-
Elevated adiponectin expression promotes adipose tissue vascularity under conditions of diet-induced obesity.Metabolism. 2013 Dec;62(12):1730-8. doi: 10.1016/j.metabol.2013.07.010. Epub 2013 Aug 28. Metabolism. 2013. PMID: 23993424 Free PMC article.
-
Intracellular ATP in balance of pro- and anti-inflammatory cytokines in adipose tissue with and without tissue expansion.Int J Obes (Lond). 2017 Apr;41(4):645-651. doi: 10.1038/ijo.2017.3. Epub 2017 Feb 7. Int J Obes (Lond). 2017. PMID: 28074058 Free PMC article.
-
Insight into the role of dermal white adipose tissue loss in dermal fibrosis.J Cell Physiol. 2022 Jan;237(1):169-177. doi: 10.1002/jcp.30552. Epub 2021 Oct 5. J Cell Physiol. 2022. PMID: 34608987 Review.
Cited by
-
Resistance training mitigates hepato-cardiac changes and muscle mitochondrial protein reductions in rats with diet-induced obesity.Heliyon. 2021 Nov 14;7(11):e08374. doi: 10.1016/j.heliyon.2021.e08374. eCollection 2021 Nov. Heliyon. 2021. PMID: 34841103 Free PMC article.
-
The new marker YKL-40, a molecule related to inflammation, is associated with cardiovascular events in stable haemodialysis patients.Clin Kidney J. 2019 May 20;13(2):172-178. doi: 10.1093/ckj/sfz056. eCollection 2020 Apr. Clin Kidney J. 2019. PMID: 32296521 Free PMC article.
-
Visceral adipose tissue remodeling in pancreatic ductal adenocarcinoma cachexia: the role of activin A signaling.Sci Rep. 2022 Jan 31;12(1):1659. doi: 10.1038/s41598-022-05660-7. Sci Rep. 2022. PMID: 35102236 Free PMC article.
-
Skin Cell and Tissue Responses to Cross-Linked Hyaluronic Acid in Low-Grade Inflammatory Conditions.Int J Inflam. 2023 Aug 26;2023:3001080. doi: 10.1155/2023/3001080. eCollection 2023. Int J Inflam. 2023. PMID: 37663889 Free PMC article.
-
Comparative analysis of cutaneous features of psoriasis in acute and chronic imiquimod-induced mouse models.Sci Rep. 2025 Jul 23;15(1):26834. doi: 10.1038/s41598-025-12111-6. Sci Rep. 2025. PMID: 40702143 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

