Safety and efficacy of n-3 fatty acid-based parenteral nutrition in patients with obstructive jaundice: a propensity-matched study
- PMID: 30006616
- PMCID: PMC6085574
- DOI: 10.1038/s41430-018-0256-1
Safety and efficacy of n-3 fatty acid-based parenteral nutrition in patients with obstructive jaundice: a propensity-matched study
Abstract
Background: It is reported that lipid emulsion enriched in n-3 fatty acids (FAs) helps us to improve postoperative recovery for surgical patients with biliary tract disease. Its role for postoperative patients with obstructive jaundice is as yet unclear. The object of this study was to evaluate the safety and efficacy of n-3 fatty acid-based parenteral nutrition (PN) for patients with obstructive jaundice following surgical procedures.
Methods: Data were collected from patients with obstructive jaundice who received PN, including n-3 PUFA-enriched lipid emulsions and standard non-enriched lipid emulsions (e.g., soybean oil). We then calculated a propensity score, the probability of receiving different PN, by the propensity score matched (PSM) method. After matching, we compared isonitrogenous total PN with 20% Structolipid and 10% n-3 fatty acid (Omegaven, Fresenius-Kabi, Germany) (treatment group) to Structolipid alone (control group) for 5 days postoperatively, in the absence of enteral nutrition.
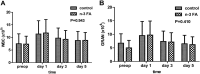
Results: Before the propensity score matching, there were 226 patients enrolled. After propensity score stratification, 108 cases remained, and all covariates were balanced. Among matched patients with PN, patients in the control group were at a higher risk for long-term jaundice recovery (12.9 ± 8.5 VS 16.4 ± 7.9 P = 0.029), lower velocity of reduction in jaundice (P = 0.045), and lower pre-albumin (P = 0.002). No significant difference as found in terms of comorbidities, white blood cell (WBC), albumin and other aspects.
Conclusion: PN with n-3 PUFA-enriched lipid emulsions was safe and effective in accelerating jaundice recovery for patients after surgical procedures. This trial was registered at clinicaltrials.gov as NCT03376945.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Supplementation of omega-3 fatty acids in parenteral nutrition beneficially alters phospholipid fatty acid pattern.JPEN J Parenter Enteral Nutr. 2007 Jan-Feb;31(1):12-7. doi: 10.1177/014860710703100112. JPEN J Parenter Enteral Nutr. 2007. PMID: 17202435 Clinical Trial.
-
n-3 fatty acid-based parenteral nutrition improves postoperative recovery for cirrhotic patients with liver cancer: A randomized controlled clinical trial.Clin Nutr. 2017 Oct;36(5):1239-1244. doi: 10.1016/j.clnu.2016.08.002. Epub 2016 Aug 31. Clin Nutr. 2017. PMID: 27614675 Clinical Trial.
-
Four-week parenteral nutrition using a third generation lipid emulsion (SMOFlipid)--a double-blind, randomised, multicentre study in adults.Clin Nutr. 2013 Apr;32(2):224-31. doi: 10.1016/j.clnu.2012.06.011. Epub 2012 Jul 12. Clin Nutr. 2013. PMID: 22796064 Clinical Trial.
-
ω-3 Fatty-Acid Enriched Parenteral Nutrition in Hospitalized Patients: Systematic Review With Meta-Analysis and Trial Sequential Analysis.JPEN J Parenter Enteral Nutr. 2020 Jan;44(1):44-57. doi: 10.1002/jpen.1672. Epub 2019 Jun 27. JPEN J Parenter Enteral Nutr. 2020. PMID: 31250474 Free PMC article.
-
What is the current role for parenteral lipid emulsions containing omega-3 fatty acids in infants with short bowel syndrome?Minerva Pediatr. 2009 Jun;61(3):263-72. Minerva Pediatr. 2009. PMID: 19461570 Review.
Cited by
-
Body composition as a prognostic factor in cholangiocarcinoma: a meta-analysis.Nutr J. 2024 Nov 15;23(1):145. doi: 10.1186/s12937-024-01037-w. Nutr J. 2024. PMID: 39548545 Free PMC article.
-
The roles of the glucagon-like peptide-2 and the serum TGF-β1 levels in the intestinal barrier and immune functions in rats with obstructive jaundice.Am J Transl Res. 2021 Sep 15;13(9):10449-10458. eCollection 2021. Am J Transl Res. 2021. PMID: 34650714 Free PMC article.
-
n-3 Polyunsaturated Fatty Acid Supplementation Affects Oxidative Stress Marker Levels in Patients with Type II Intestinal Failure: A Randomized Double Blind Trial.Antioxidants (Basel). 2023 Jul 26;12(8):1493. doi: 10.3390/antiox12081493. Antioxidants (Basel). 2023. PMID: 37627489 Free PMC article.
-
Does inclusion of bioactive n-3 PUFAs in parenteral nutrition benefit postoperative patients undergoing liver surgery? A systematic review and meta-analysis of randomised control trials.BMJ Open. 2023 Sep 14;13(9):e066171. doi: 10.1136/bmjopen-2022-066171. BMJ Open. 2023. PMID: 37709313 Free PMC article.
References
-
- Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–20. doi: 10.1097/00003246-199908000-00001. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

