A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice
- PMID: 30007419
- PMCID: PMC6092020
- DOI: 10.1016/j.cell.2018.06.042
A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice
Abstract
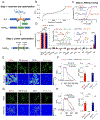
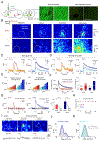
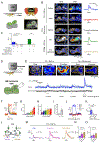
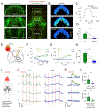
Dopamine (DA) is a central monoamine neurotransmitter involved in many physiological and pathological processes. A longstanding yet largely unmet goal is to measure DA changes reliably and specifically with high spatiotemporal precision, particularly in animals executing complex behaviors. Here, we report the development of genetically encoded GPCR-activation-based-DA (GRABDA) sensors that enable these measurements. In response to extracellular DA, GRABDA sensors exhibit large fluorescence increases (ΔF/F0 ∼90%) with subcellular resolution, subsecond kinetics, nanomolar to submicromolar affinities, and excellent molecular specificity. GRABDA sensors can resolve a single-electrical-stimulus-evoked DA release in mouse brain slices and detect endogenous DA release in living flies, fish, and mice. In freely behaving mice, GRABDA sensors readily report optogenetically elicited nigrostriatal DA release and depict dynamic mesoaccumbens DA signaling during Pavlovian conditioning or during sexual behaviors. Thus, GRABDA sensors enable spatiotemporally precise measurements of DA dynamics in a variety of model organisms while exhibiting complex behaviors.
Keywords: GPCR; GRAB(DA); dopamine; sensor.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare competing financial interests. F.S., J.Z., M.J. and Y.L. have filed patent applications whose value might be affected by this publication.
Figures



References
-
- Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, and Heberlein U (2000). Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol 10, 187–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

