Soluble antigen arrays disarm antigen-specific B cells to promote lasting immune tolerance in experimental autoimmune encephalomyelitis
- PMID: 30007842
- PMCID: PMC6117839
- DOI: 10.1016/j.jaut.2018.06.006
Soluble antigen arrays disarm antigen-specific B cells to promote lasting immune tolerance in experimental autoimmune encephalomyelitis
Abstract
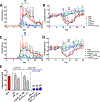
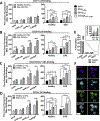
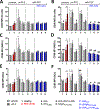
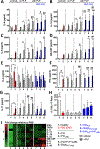
Autoreactive lymphocytes that escape central immune tolerance may be silenced via an endogenous peripheral tolerance mechanism known as anergy. Antigen-specific therapies capable of inducing anergy may restore patients with autoimmune diseases to a healthy phenotype while avoiding deleterious side effects associated with global immunosuppression. Inducing anergy in B cells may be a particularly potent intervention, as B cells can contribute to autoimmune diseases through multiple mechanisms and offer the potential for direct antigen-specific targeting through the B cell receptor (BCR). Our previous results suggested autoreactive B cells may be silenced by multivalent 'soluble antigen arrays' (SAgAs), which are polymer conjugates displaying multiple copies of autoantigen with or without a secondary peptide that blocks intracellular cell-adhesion molecule-1 (ICAM-1). Here, key therapeutic molecular properties of SAgAs were identified and linked to the immunological mechanism through comprehensive cellular and in vivo analyses. We determined non-hydrolyzable 'cSAgAs' displaying multivalent 'click'-conjugated antigen more potently suppressed experimental autoimmune encephalomyelitis (EAE) compared to hydrolyzable SAgAs capable of releasing conjugated antigen. cSAgAs restored a healthy phenotype in disease-specific antigen presenting cells (APCs) by inducing an anergic response in B cells and a subset of B cells called autoimmune-associated B cells (ABCs) that act as potent APCs in autoimmune disease. Accompanied by a cytokine response skewed towards a Th2/regulatory phenotype, this generated an environment of autoantigenic tolerance. By identifying key therapeutic molecular properties and an immunological mechanism that drives SAgA efficacy, this work guides the design of antigen-specific immunotherapies capable of inducing anergy.
Keywords: Antigen-specific B cells; Autoimmune-associated B cells; Autoimmunity; EAE; Tolerance.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
C.J.B. is a co-founder and Chairman of the Board of Orion BioScience. Orion has licensed the rights to patents claiming soluble antigen arrays.
Figures


References
-
- Kontos S; Grimm AJ; Hubbell JA, Engineering antigen-specific immunological tolerance. Curr. Opin. Immunol 2015, 35, 80–88. - PubMed
-
- Mueller DL; Jenkins MK; Schwartz RH, Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu. Rev. Immunol 1989, 7 (1), 445–480. - PubMed
-
- Feldmann M; Steinman L, Design of effective immunotherapy for human autoimmunity. Nature 2005, 435 (7042), 612. - PubMed
-
- Miller SD; Turley DM; Podojil JR, Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat. Rev. Immunol 2007, 7 (9), 665. - PubMed
-
- Mueller DL, Mechanisms maintaining peripheral tolerance. Nat. Immunol 2010,11 (1), 21–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

