A Dedicated Population for Reward Coding in the Hippocampus
- PMID: 30008297
- PMCID: PMC7023678
- DOI: 10.1016/j.neuron.2018.06.008
A Dedicated Population for Reward Coding in the Hippocampus
Abstract
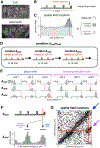
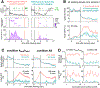
The hippocampus plays a critical role in goal-directed navigation. Across different environments, however, hippocampal maps are randomized, making it unclear how goal locations could be encoded consistently. To address this question, we developed a virtual reality task with shifting reward contingencies to distinguish place versus reward encoding. In mice performing the task, large-scale recordings in CA1 and subiculum revealed a small, specialized cell population that was only active near reward yet whose activity could not be explained by sensory cues or stereotyped reward anticipation behavior. Across different virtual environments, most cells remapped randomly, but reward encoding consistently arose from a single pool of cells, suggesting that they formed a dedicated channel for reward. These observations represent a significant departure from the current understanding of CA1 as a relatively homogeneous ensemble without fixed coding properties and provide a new candidate for the cellular basis of goal memory in the hippocampus.
Keywords: CA1; hippocampus; navigation; place cells; place fields; reward; subiculum; virtual reality.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare no competing interests.
Figures




Comment in
-
Treasure hunt.Nat Rev Neurosci. 2018 Aug;19(8):443. doi: 10.1038/s41583-018-0042-z. Nat Rev Neurosci. 2018. PMID: 29991694 No abstract available.
-
Are We There Yet? Identification of Reward-Selective Cells in the Hippocampus.Neuron. 2018 Jul 11;99(1):7-10. doi: 10.1016/j.neuron.2018.06.037. Neuron. 2018. PMID: 30001513
References
-
- Andersen P, Morris R, Amaral DG, Bliss T, & O’Keefe J (2007). The Hippocampus Book. (Oxford University Press, USA: ).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

