Chewing during prenatal stress prevents prenatal stress-induced suppression of neurogenesis, anxiety-like behavior and learning deficits in mouse offspring
- PMID: 30008596
- PMCID: PMC6036092
- DOI: 10.7150/ijms.25281
Chewing during prenatal stress prevents prenatal stress-induced suppression of neurogenesis, anxiety-like behavior and learning deficits in mouse offspring
Abstract
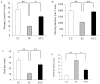
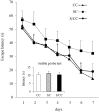
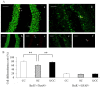
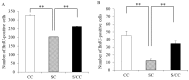
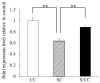
Prenatal stress (PS) induces learning deficits and anxiety-like behavior in mouse pups by increasing corticosterone levels in the dam. We examined the effects of maternal chewing during PS on arginine vasopressin (AVP) mRNA expression in the dams and on neurogenesis, brain-derived neurotrophic factor (BDNF) mRNA expression, learning deficits and anxiety-like behavior in the offspring. Mice were divided into control, stress and stress/chewing groups. Pregnant mice were exposed to restraint stress beginning on day 12 of pregnancy and continuing until delivery. Mice in the stress/chewing group were given a wooden stick to chew during restraint stress. PS significantly increased AVP mRNA expression in the paraventricular nucleus (PVN) of the hypothalamus in the dams. PS also impaired learning ability, suppressed neurogenesis and BDNF mRNA expression in the hippocampus, and induced anxiety-like behavior in the offspring. Chewing during PS prevented the PS-induced increase in AVP mRNA expression of the PVN in the dams. Chewing during PS significantly attenuated the PS-induced learning deficits, anxiety-like behavior, and suppression of neurogenesis and BDNF mRNA expression in the hippocampus of the offspring. Chewing during PS prevented the increase in plasma corticosterone in the dam by inhibiting the hypothalamic-pituitary-adrenal axis activity, and attenuated the attenuated the PS-induced suppression of neurogenesis and BDNF expression in the hippocampus of the pups, thereby ameliorating the PS-induced learning deficits and anxiety-like behavior. Chewing during PS is an effective stress-coping method for the dam to prevent PS-induced deficits in learning ability and anxiety-like behavior in the offspring.
Keywords: Anxiety-like behavior; BDNF; Chewing; Learning ability; Neurogenesis; Prenatal stress.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Bustamante C, Bilbao P, Contrerans W. et al. Effects of prenatal stress and exercise on dentate granule cells maturation and spatial memory in adolescent mice. Int J Dev Neuroscience. 2010;28:605–609. - PubMed
-
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

