B Cell-Related Circulating MicroRNAs With the Potential Value of Biomarkers in the Differential Diagnosis, and Distinguishment Between the Disease Activity and Lupus Nephritis for Systemic Lupus Erythematosus
- PMID: 30008716
- PMCID: PMC6033964
- DOI: 10.3389/fimmu.2018.01473
B Cell-Related Circulating MicroRNAs With the Potential Value of Biomarkers in the Differential Diagnosis, and Distinguishment Between the Disease Activity and Lupus Nephritis for Systemic Lupus Erythematosus
Abstract
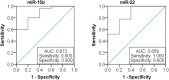
Our understanding of circulating microRNAs (miRNAs) related to systemic lupus erythematosus (SLE) remains very limited. In this study, we screened SLE-specific miRNAs in plasma from 42 B cell-related miRNAs by using miRNA PCR Array. The selected miRNAs were first confirmed in plasma samples from 50 SLE patients, 16 rheumatoid arthritis (RA) patients, and 20 healthy donors using qRT-PCR. We then investigated the relationship between expressions of the selected miRNAs and SLE clinical indicators. As a result, 14 miRNAs (miR-103, miR-150, miR-20a, miR-223, miR-27a, miR-15b, miR-16, miR-181a, miR-19b, miR-22, miR-23a, miR-25, miR-92a, and miR-93) were significantly decreased in the plasma of SLE patients compared with healthy controls (P < 0.05) and could act as the diagnostic signature to distinguish SLE patients from healthy donors. Six miRNAs (miR-92a, miR-27a, miR-19b, miR-23a, miR-223, and miR-16) expressed in plasma were significantly lower in SLE patients than in RA patients (P < 0.05), revealing the potentially diagnostic signature to distinguish SLE patients from RA patients. Furthermore, the downregulated expression of miR-19b, miR-25, miR-93, and miR-15b was associated with SLE disease activity (P < 0.05) while miR-15b and miR-22 expressions were significantly lower in SLE patients with low estimate glomerular filtration rate (eGFR < 60 ml/min/1.73 m2) (P < 0.05). The diagnostic potential of miR-15b for SLE disease activity and lupus nephritis (LN) with low eGFR was validated on an independent validation set with 69 SLE patients and a cross-validation set with 80 SLE patients. In summary, the signature of circulating miRNAs will provide novel biomarkers for the diagnosis of SLE and evaluation of disease activity and LN.
Keywords: biomarker; circulating microRNA; diagnosis; miR-15b; systemic lupus erythematosus.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

