Decreased argininosuccinate synthetase expression in Thai patients with cholangiocarcinoma and the effects of ADI-PEG20 treatment in CCA cell lines
- PMID: 30008833
- PMCID: PMC6036342
- DOI: 10.3892/ol.2018.8807
Decreased argininosuccinate synthetase expression in Thai patients with cholangiocarcinoma and the effects of ADI-PEG20 treatment in CCA cell lines
Abstract
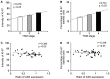
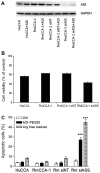
Cholangiocarcinoma (CCA) is a severe cancer with poor prognosis. The aim of the present study was to explore the expression of argininosuccinate synthetase (ASS), as well as the possibility of using pegylated arginine deiminase (ADI-PEG20) for the treatment of CCA. ASS expression was determined in CCA specimens from 40 patients in Thailand. Immunohistochemical detection of ASS and determination of the proliferative index, Ki-67, were carried out in paraffin-embedded sections of these specimens, as well as in two CCA cell lines, HuCCA and RmCCA-1, derived from CCA samples from patients in Thailand. In total, ~45% of the CCA specimens had low ASS expression, and the level of expression was significantly negatively associated with cell differentiation (P<0.05) and Ki-67 expression (P<0.05). The level of ASS expression in tumor cells was significantly lower than that in non-tumor cells (1.3-fold, P<0.05). The HuCCA cell line had significantly lower levels (P<0.05) of ASS expression at the mRNA and protein levels relative to those of normal human immortalized fibroblast cells (BJ-1). By contrast, the RmCCA-1 cell line showed no significant difference. In addition, the effects of ADI-PEG20 on growth inhibition, apoptosis and cell cycle arrest were determined in HuCCA and RmCCA-1 cells. ADI-PEG20 treatment reduced cell viability and cell proliferation in the two CCA cell lines, though it had no effect in immortalized BJ-1 cells. Furthermore, ADI-PEG20 treatment significantly increased G0/G1 cell cycle arrest in HuCCA, though not in RmCCA-1 cells. ASS silencing in the RmCCA-1 cell line significantly enhanced its sensitivity to ADI-PEG20 treatment. Results from the in vitro study demonstrated that ADI-PEG20 has antitumor activity against CCA with low ASS expression.
Keywords: Ki-67; arginine deprivation; argininosuccinate synthetase; cholangiocarcinoma; pegylated arginine deiminase.
Figures





Similar articles
-
The Combination of Arginine Deprivation and 5-Fluorouracil Improves Therapeutic Efficacy in Argininosuccinate Synthetase Negative Hepatocellular Carcinoma.Int J Mol Sci. 2017 Jun 1;18(6):1175. doi: 10.3390/ijms18061175. Int J Mol Sci. 2017. PMID: 28587170 Free PMC article.
-
Arginine deiminase PEG20 inhibits growth of small cell lung cancers lacking expression of argininosuccinate synthetase.Br J Cancer. 2012 Jan 17;106(2):324-32. doi: 10.1038/bjc.2011.524. Epub 2011 Dec 1. Br J Cancer. 2012. PMID: 22134507 Free PMC article.
-
Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis.Cancer Res. 2009 Jan 15;69(2):700-8. doi: 10.1158/0008-5472.CAN-08-3157. Cancer Res. 2009. PMID: 19147587 Free PMC article.
-
Targeted cellular metabolism for cancer chemotherapy with recombinant arginine-degrading enzymes.Oncotarget. 2010 Aug;1(4):246-51. doi: 10.18632/oncotarget.135. Oncotarget. 2010. PMID: 21152246 Free PMC article. Review.
-
Arginine deprivation as a targeted therapy for cancer.Curr Pharm Des. 2008;14(11):1049-57. doi: 10.2174/138161208784246199. Curr Pharm Des. 2008. PMID: 18473854 Free PMC article. Review.
Cited by
-
Elevated ITGA2 expression promotes collagen type I-induced clonogenic growth of intrahepatic cholangiocarcinoma.Sci Rep. 2022 Dec 27;12(1):22429. doi: 10.1038/s41598-022-26747-1. Sci Rep. 2022. PMID: 36575207 Free PMC article.
-
Multifaceted Aspects of Metabolic Plasticity in Human Cholangiocarcinoma: An Overview of Current Perspectives.Cells. 2020 Mar 3;9(3):596. doi: 10.3390/cells9030596. Cells. 2020. PMID: 32138158 Free PMC article. Review.
-
Amino Acid Degrading Enzymes and Autophagy in Cancer Therapy.Front Pharmacol. 2021 Jan 11;11:582587. doi: 10.3389/fphar.2020.582587. eCollection 2020. Front Pharmacol. 2021. PMID: 33510635 Free PMC article. Review.
-
Arginase: Mechanisms and Clinical Application in Hematologic Malignancy.Front Oncol. 2022 Jun 23;12:905893. doi: 10.3389/fonc.2022.905893. eCollection 2022. Front Oncol. 2022. PMID: 35814439 Free PMC article. Review.
-
Arginine Is a Novel Drug Target for Arginine Decarboxylase in Human Colorectal Cancer Cells.Int J Mol Sci. 2023 Sep 6;24(18):13741. doi: 10.3390/ijms241813741. Int J Mol Sci. 2023. PMID: 37762044 Free PMC article.
References
-
- Parkin DM, Ohshima H, Srivatanakul P, Vatanasapt V. Cholangiocarcinoma: Epidemiology, mechanisms of carcinogenesis and prevention. Cancer Epidemiol Biomarkers Prev. 1993;2:537–544. - PubMed
-
- Mosadeghi S, Liu B, Bhuket T, Wong RJ. Sex-specific and race/ethnicity-specific disparities in cholangiocarcinoma incidence and prevalence in the USA: An updated analysis of the 2000–2011 surveillance, epidemiology and end results registry. Hepatol Res. 2016;46:669–677. doi: 10.1111/hepr.12605. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
