Altered dynamics of neurovascular coupling in CADASIL
- PMID: 30009197
- PMCID: PMC6043774
- DOI: 10.1002/acn3.574
Altered dynamics of neurovascular coupling in CADASIL
Abstract
Background and objective: Neurovascular coupling is the complex biological process that underlies use-dependent increases in blood flow in response to neural activation. Neurovascular coupling was investigated at the early stage of CADASIL, a genetic paradigm of ischemic small vessel disease.
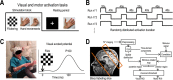
Methods: Functional hyperemia and evoked potentials during 20- and 40-sec visual and motor stimulations were monitored simultaneously using arterial spin labeling-functional magnetic resonance imaging (ASL-fMRI) and electroencephalography.
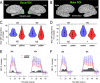
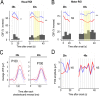
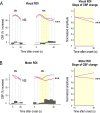
Results: Cortical functional hyperemia differed significantly between 19 patients and 19 healthy individuals, whereas evoked potentials were unaltered. Functional hyperemia dynamics, assessed using the difference in the slope of the response curve between 15 and 30 sec, showed a time-shifted decrease in the response to 40-sec neural stimulations in CADASIL patients. These results were replicated in a second cohort of 10 patients and 10 controls and confirmed in the whole population.
Interpretation: Alterations of neurovascular coupling occur early in CADASIL and can be assessed by ASL-fMRI using a simple marker of vascular dysfunction.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

