Injectable DaxibotulinumtoxinA in Cervical Dystonia: A Phase 2 Dose-Escalation Multicenter Study
- PMID: 30009213
- PMCID: PMC6032882
- DOI: 10.1002/mdc3.12613
Injectable DaxibotulinumtoxinA in Cervical Dystonia: A Phase 2 Dose-Escalation Multicenter Study
Abstract
Background: Injectable daxibotulinumtoxinA (an investigational botulinum toxin, RT002) may offer a more prolonged duration of response-and therefore less frequent dosing-than onabotulinumtoxinA.
Objectives: To perform a phase 2, open-label, dose-escalation study to assess the efficacy and safety of daxibotulinumtoxinA in cervical dystonia.
Methods: Subjects with moderate-to-severe isolated cervical dystonia were enrolled in sequential cohorts to receive a single open-label, intramuscular dose of injectable daxibotulinumtoxinA of up to 200 U (n = 12), 200-300 U (n = 12), or 300-450 U (n = 13; https://clinicaltrials.gov identifier NCT02706795).
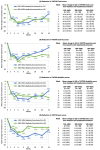
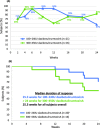
Results: Overall, 33/37 enrollees completed the trial. DaxibotulinumtoxinA was associated with mean reductions in Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS)-Total score of 16.8 (38%) at week 4, 21.3 (50%) at week 6, and 12.8 (30%) at week 24. The proportion of subjects who were responders (achieved ≥ 20% reduction in TWSTRS-Total score) was 94% at week 6 and 68% at week 24. The median duration of response (time until > 20% of the improvement in TWSTRS-Total score achieved at week 4 was no longer retained or re-treatment was needed) was 25.3 weeks (95% CI, 20.14-26.14 weeks). There were no serious adverse events and there was no apparent dose-related increase in the incidence of adverse events. The most common treatment-related adverse events were dysphagia (14%) and injection site erythema (8%).
Conclusions: Preliminary assessments suggest that injectable daxibotulinumtoxinA at doses up to 450 U is well tolerated and may offer prolonged efficacy in the treatment of cervical dystonia. Further studies involving larger numbers of patients are now warranted.
Keywords: CDIP; TWSTRS; botulinum toxin; cervical dystonia; daxibotulinumtoxinA.
Figures

Similar articles
-
Efficacy and Safety of DaxibotulinumtoxinA for Injection in Cervical Dystonia: ASPEN-1 Phase 3 Randomized Controlled Trial.Neurology. 2024 Feb 27;102(4):e208091. doi: 10.1212/WNL.0000000000208091. Epub 2024 Jan 31. Neurology. 2024. PMID: 38295339 Free PMC article. Clinical Trial.
-
Long-Term Safety and Efficacy of Repeat Treatments with DaxibotulinumtoxinA in Cervical Dystonia: Results from the ASPEN-Open-Label Study.Mov Disord Clin Pract. 2025 May 29. doi: 10.1002/mdc3.70104. Online ahead of print. Mov Disord Clin Pract. 2025. PMID: 40439027
-
Efficacy and safety of abobotulinumtoxinA liquid formulation in cervical dystonia: A randomized-controlled trial.Mov Disord. 2016 Nov;31(11):1649-1657. doi: 10.1002/mds.26760. Epub 2016 Sep 21. Mov Disord. 2016. PMID: 27653448 Clinical Trial.
-
Overview of DaxibotulinumtoxinA for Injection: A Novel Formulation of Botulinum Toxin Type A.Drugs. 2021 Dec;81(18):2091-2101. doi: 10.1007/s40265-021-01631-w. Epub 2021 Nov 17. Drugs. 2021. PMID: 34787840 Free PMC article. Review.
-
Botulinum toxin B: a review of its therapeutic potential in the management of cervical dystonia.Drugs. 2002;62(4):705-22. doi: 10.2165/00003495-200262040-00011. Drugs. 2002. PMID: 11893235 Review.
Cited by
-
Botulinum Toxin: An Update on Pharmacology and Newer Products in Development.Toxins (Basel). 2021 Jan 14;13(1):58. doi: 10.3390/toxins13010058. Toxins (Basel). 2021. PMID: 33466571 Free PMC article. Review.
-
Retroform Cervical Dystonia: Target Muscle Selection and Efficacy of Botulinum Toxin Injection.Front Neurol. 2022 Jul 26;13:952456. doi: 10.3389/fneur.2022.952456. eCollection 2022. Front Neurol. 2022. PMID: 35959387 Free PMC article.
-
Prospect of cell penetrating peptides in stem cell tracking.Stem Cell Res Ther. 2021 Aug 14;12(1):457. doi: 10.1186/s13287-021-02522-3. Stem Cell Res Ther. 2021. PMID: 34391472 Free PMC article. Review.
-
Treatment of dystonia and tics.Clin Park Relat Disord. 2019 Dec 4;2:12-19. doi: 10.1016/j.prdoa.2019.11.005. eCollection 2020. Clin Park Relat Disord. 2019. PMID: 34316614 Free PMC article. Review.
-
A randomized, double-blind, placebo-controlled trial of DaxibotulinumtoxinA for Injection for the treatment of upper limb spasticity in adults after stroke or traumatic brain injury.PM R. 2025 Feb;17(2):126-136. doi: 10.1002/pmrj.13258. Epub 2024 Oct 1. PM R. 2025. PMID: 39352144 Free PMC article. Clinical Trial.
References
-
- Jankovic J. Botulinum toxin: State of the art. Mov Disord 2017;32:1131–1138. - PubMed
-
- Carruthers J, Solish N, Humphrey S, et al. Injectable daxibotulinumtoxinA for the treatment of glabellar lines: a phase 2, randomized, dose‐ranging, double‐blind, multicenter comparison with onabotulinumtoxinA and placebo. Dermatol Surg 2017;43:1321–1331. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

