Inhibition of androgen receptor transactivation function by adenovirus type 12 E1A undermines prostate cancer cell survival
- PMID: 30009471
- PMCID: PMC6424568
- DOI: 10.1002/pros.23689
Inhibition of androgen receptor transactivation function by adenovirus type 12 E1A undermines prostate cancer cell survival
Abstract
Background: Mutations or truncation of the ligand-binding domain (LBD) of androgen receptor (AR) underlie treatment resistance for prostate cancer (PCa). Thus, targeting the AR N-terminal domain (NTD) could overcome such resistance.
Methods: Luciferase reporter assays after transient transfection of various DNA constructs were used to assess effects of E1A proteins on AR-mediated transcription. Immunofluorescence microscopy and subcellular fractionation were applied to assess intracellular protein localization. Immunoprecipitation and mammalian two-hybrid assays were used to detect protein-protein interactions. qRT-PCR was employed to determine RNA levels. Western blotting was used to detect protein expression in cells. Effects of adenoviruses on prostate cancer cell survival were evaluated with CellTiter-Glo assays.
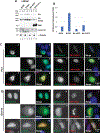
Results: Adenovirus 12 E1A (E1A12) binds specifically to the AR. Interestingly, the full-length E1A12 (266 aa) preferentially binds to full-length AR, while the small E1A12 variant (235 aa) interacts more strongly with AR-V7. E1A12 promotes AR nuclear translocation, likely through mediating intramolecular AR NTD-LBD interactions. In the nucleus, AR and E1A12 co-expression in AR-null PCa cells results in E1A12 redistribution from nuclear foci containing CBX4 (also known as Pc2), suggesting a preferential AR-E1A12 interaction over other E1A12 interactors. E1A12 represses AR-mediated transcription in reporter gene assays and endogenous AR target genes such as ATAD2 and MYC in AR-expressing PCa cells. AR-expressing PCa cells are more sensitive to death induced by a recombinant adenovirus expressing E1A12 (Ad-E1A12) than AR-deficient PCa cells, which could be attributed to the increased viral replication promoted by androgen stimulation. Targeting the AR by E1A12 promotes apoptosis in PCa cells that express the full-length AR or C-terminally truncated AR variants. Importantly, inhibition of mTOR signaling that blocks the expression of anti-apoptotic proteins markedly augments Ad-E1A12-induced apoptosis of AR-expressing cells. Mechanistically, Ad-E1A12 infection triggers apoptotic response while activating the PI3K-AKT-mTOR signaling axis; thus, mTOR inhibition enhances apoptosis in AR-expressing PCa cells infected by Ad-E1A12.
Conclusion: Ad12 E1A inhibits AR-mediated transcription and suppresses PCa cell survival, suggesting that targeting the AR by E1A12 might have therapeutic potential for treating advanced PCa with heightened AR signaling.
Keywords: AR splice variants (AR-Vs); PI3K-kinase (PI3K)-Akt-mTOR signaling; androgen receptor; prostate cancer gene therapy; transcriptional regulation.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICTS OF INTEREST
There are no conflicts of interest to disclose.
Figures




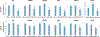


References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63(1):11–30. - PubMed
-
- van de Wijngaart DJ, Dubbink HJ, van Royen ME, Trapman J, Jenster G. Androgen receptor coregulators: recruitment via the coactivator binding groove. Mol Cell Endocrinol 2012;352(1–2):57–69. - PubMed
-
- McEwan IJ. Intrinsic disorder in the androgen receptor: identification, characterisation and drugability. Mol Biosyst 2012;8(1):82–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

