Reduced-Intensity Conditioning and Dual T Lymphocyte Suppression with Antithymocyte Globulin and Post-Transplant Cyclophosphamide as Graft-versus-Host Disease Prophylaxis in Haploidentical Hematopoietic Stem Cell Transplants for Hematological Malignancies
- PMID: 30009980
- PMCID: PMC7110605
- DOI: 10.1016/j.bbmt.2018.07.008
Reduced-Intensity Conditioning and Dual T Lymphocyte Suppression with Antithymocyte Globulin and Post-Transplant Cyclophosphamide as Graft-versus-Host Disease Prophylaxis in Haploidentical Hematopoietic Stem Cell Transplants for Hematological Malignancies
Abstract
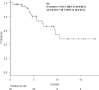
Haploidentical hematopoietic stem cell transplantation (haploHSCT) with conditioning regimens using post-transplant cyclophosphamide (PTCy) for peripheral blood stem cell (PBSC) grafts is limited by comparably higher rates of acute and chronic graft-versus-host disease (GVHD). Antithymocyte globulin (ATG) may mitigate this risk. We evaluated haploHSCT after reduced-intensity conditioning (RIC) with ATG, PTCy, and cyclosporine to prevent rejection and GVHD. Fifty adults underwent haploHSCT from August 2016 to February 2018. RIC included fludarabine (30 mg/m2/day on days -5 to -2), busulfan (3.2 mg/m2/day on days -3 and -2), and total body irradiation (200 cGy) on day -1. Unmanipulated PBSCs were infused on day 0. GVHD prophylaxis included ATG (4.5 mg/kg over days -3 to -1), PTCy (50 mg/kg/day on days +3 and +4), and cyclosporine from day +5. Median age was 56 years (range, 22 to 70 years); 25 (73.5%) patients were in first complete remission (CR1), 5 (14.7%) were in second complete remission (CR2), and 8 (23.5%) had active disease. Median time to neutrophil engraftment was 16 days (range, 8 to 43 days). At day +100, the cumulative incidence of acute GVHD of any grade, and grades III to IV was 38.3% and 5.2%, respectively. Mild chronic GVHD was seen in 15.5%. Cytomegalovirus (CMV) reactivation occurred in 37 (74%) cases and CMV disease occurred in 4 (11.5%) cases. Epstein-Barr virus (EBV) reactivation occurred in 21 (61.8%) patients. The incidence of histologically confirmed post-transplantation lymphoproliferative disorder (PTLD) was 5.8%. Four patients received rituximab. There were no CMV, EBV, or PTLD-related deaths. Six-month and 1-year overall survival (OS), cumulative incidence of relapse (CIR), and nonrelapse mortality (NRM) were 73.9%, 10.2%, and 19.4%, respectively, and 48.1%, 16% and 38.2%, respectively. Infection was the most common cause of death (18%). Unmanipulated haploidentical PBSC transplantation following RIC with ATG, PTCy, and cyclosporine as a GVHD prevention strategy results in low rates of acute and chronic GVHD.
Keywords: Antithymocyte globulin; Dual T cell suppression; Haploidentical hematopoietic stem cell transplantation; Post-transplant cyclophosphamide.
Copyright © 2018. Published by Elsevier Inc.
Figures



References
-
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–1826. - PubMed
-
- Besse K, Maiers M, Confer D, Albrecht M. On modeling human leukocyte antigen-identical sibling match probability for allogeneic hematopoietic cell transplantation: estimating the need for an unrelated donor source. Biol Blood Marrow Transplant. 2016;22(3):410–417. - PubMed
-
- Deotare U, Atenafu EG, Loach D. Reduction of severe acute graft-versus-host disease using a combination of pre transplant anti-thymocyte globulin and post-transplant cyclophosphamide in matched unrelated donor transplantation. Bone Marrow Transplant. 2018;53(3):361–365. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

