Optimal bone fracture repair requires 24R,25-dihydroxyvitamin D3 and its effector molecule FAM57B2
- PMID: 30010626
- PMCID: PMC6063485
- DOI: 10.1172/JCI98093
Optimal bone fracture repair requires 24R,25-dihydroxyvitamin D3 and its effector molecule FAM57B2
Abstract
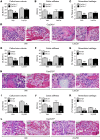
The biological activity of 24R,25-dihydroxyvitamin D3 [24R,25(OH)2D3] remains controversial, but it has been suggested that it contributes to fracture healing. Cyp24a1-/- mice, synthesizing no 24R,25(OH)2D3, show suboptimal endochondral ossification during fracture repair, with smaller callus and reduced stiffness. These defects were corrected by 24R,25(OH)2D3 treatment, but not by 1,25-dihydroxyvitamin D3. Microarrays with Cyp24a1-/- callus mRNA identified FAM57B2 as a mediator of the 24R,25(OH)2D3 effect. FAM57B2 produced lactosylceramide (LacCer) upon specific binding of 24R,25(OH)2D3. Fam57b inactivation in chondrocytes (Col2-Cre Fam57bfl/fl) phenocopied the callus formation defect of Cyp24a1-/- mice. LacCer or 24R,25(OH)2D3 injections restored callus volume, stiffness, and mineralized cartilage area in Cyp24a1-null mice, but only LacCer rescued Col2-Cre Fam57bfl/fl mice. Gene expression in callus tissue suggested that the 24R,25(OH)2D3/FAM57B2 cascade affects cartilage maturation. We describe a previously unrecognized pathway influencing endochondral ossification during bone repair through LacCer production upon binding of 24R,25(OH)2D3 to FAM57B2. Our results identify potential new approaches to ameliorate fracture healing.
Keywords: Bone Biology; Endocrinology; Orthopedics.
Conflict of interest statement
Figures




Comment in
-
The good and the bad of vitamin D inactivation.J Clin Invest. 2018 Aug 31;128(9):3736-3738. doi: 10.1172/JCI122046. Epub 2018 Aug 6. J Clin Invest. 2018. PMID: 30080183 Free PMC article.
References
-
- Horst RL, Reinhardt TA, Reddy GS. Vitamin D metabolism. In: Feldman D, Pike JW, Glorieux FH, eds. Vitamin D. 2nd ed. San Diego: Elsevier Academic Press; 2005:15–36.
-
- St-Arnaud R. CYP24A1: structure, function, and physiological role. In: Feldman D, Pike JW, Adams JS eds. Vitamin D. 2nd ed. San Diego: Academic Press; 2011:43–56.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

