WBP2 shares a common location in mouse spermatozoa with WBP2NL/PAWP and like its descendent is a candidate mouse oocyte-activating factor
- PMID: 30010725
- PMCID: PMC6299249
- DOI: 10.1093/biolre/ioy156
WBP2 shares a common location in mouse spermatozoa with WBP2NL/PAWP and like its descendent is a candidate mouse oocyte-activating factor
Abstract
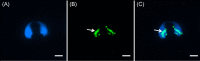
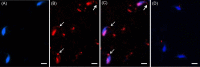
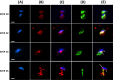
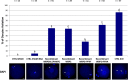
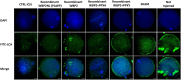
The sperm-borne oocyte-activating factor (SOAF) resides in the sperm perinuclear theca (PT). A consensus has been reached that SOAF most likely resides in the postacrosomal sheath (PAS), which is the first region of the PT to solubilize upon sperm-oocyte fusion. There are two SOAF candidates under consideration: PLCZ1 and WBP2NL. A mouse gene germline ablation of the latter showed that mice remain fertile with no observable phenotype despite the fact that a competitive inhibitor of WBP2NL, derived from its PPXY motif, blocks oocyte activation when coinjected with WBP2NL or spermatozoa. This suggested that the ortholog of WBP2NL, WBP2, containing the same domain and motifs associated with WBP2NL function, might compensate for its deficiency in oocyte activation. Our objectives were to examine whether WBP2 meets the developmental criteria established for SOAF and whether it has oocyte-activating potential. Immunoblotting detected WBP2 in mice testis and sperm and immunofluorescence localized WBP2 to the PAS and perforatorium of the PT. Immunohistochemistry of the testes revealed that WBP2 reactivity was highest in round spermatids and immunofluorescence detected WBP2 in the cytoplasmic lobe of elongating spermatids and colocalized it with the microtubular manchette during PT assembly. Microinjection of the recombinant forms of WBP2 and WBP2NL into metaphase II mouse oocytes resulted in comparable rates of oocyte activation. This study shows that WBP2 shares a similar testicular developmental pattern and location with WBP2NL and a shared ability to activate the oocyte, supporting its consideration as a mouse SOAF component that can compensate for a WBP2NL.
Figures





Similar articles
-
The postacrosomal assembly of sperm head protein, PAWP, is independent of acrosome formation and dependent on microtubular manchette transport.Dev Biol. 2007 Dec 15;312(2):471-83. doi: 10.1016/j.ydbio.2007.08.051. Epub 2007 Sep 7. Dev Biol. 2007. PMID: 17988661
-
Localisation of phospholipase Cζ1 (PLCZ1) and postacrosomal WW-binding protein (WBP2 N-terminal like) on equine spermatozoa and flow cytometry quantification of PLCZ1 and association with cleavage in vitro.Reprod Fertil Dev. 2019 Jan;31(12):1778-1792. doi: 10.1071/RD19217. Reprod Fertil Dev. 2019. PMID: 31597592
-
Is there an association between PAWP/WBP2NL sequence, expression, and distribution in sperm cells and fertilization failures in ICSI cycles?Mol Reprod Dev. 2018 Feb;85(2):163-170. doi: 10.1002/mrd.22950. Epub 2018 Jan 22. Mol Reprod Dev. 2018. PMID: 29271520
-
Sperm Factors and Oocyte Activation: Current Controversies and Considerations.Biol Reprod. 2015 Aug;93(2):50. doi: 10.1095/biolreprod.115.130609. Epub 2015 Jul 8. Biol Reprod. 2015. PMID: 26157070 Review.
-
Interactions of sperm perinuclear theca with the oocyte: implications for oocyte activation, anti-polyspermy defense, and assisted reproduction.Microsc Res Tech. 2003 Jul 1;61(4):362-78. doi: 10.1002/jemt.10350. Microsc Res Tech. 2003. PMID: 12811742 Review.
Cited by
-
WW domain binding protein 2 (WBP2) as an oncogene in breast cancer: mechanisms and therapeutic prospects-a narrative review.Gland Surg. 2022 Dec;11(12):1984-2002. doi: 10.21037/gs-22-716. Gland Surg. 2022. PMID: 36654949 Free PMC article. Review.
-
Reciprocal Regulation of Hippo and WBP2 Signalling-Implications in Cancer Therapy.Cells. 2021 Nov 11;10(11):3130. doi: 10.3390/cells10113130. Cells. 2021. PMID: 34831354 Free PMC article. Review.
-
Core Histones Are Constituents of the Perinuclear Theca of Murid Spermatozoa: An Assessment of Their Synthesis and Assembly during Spermiogenesis and Function after Gametic Fusion.Int J Mol Sci. 2021 Jul 29;22(15):8119. doi: 10.3390/ijms22158119. Int J Mol Sci. 2021. PMID: 34360885 Free PMC article.
-
Sperm Differentiation: The Role of Trafficking of Proteins.Int J Mol Sci. 2020 May 24;21(10):3702. doi: 10.3390/ijms21103702. Int J Mol Sci. 2020. PMID: 32456358 Free PMC article. Review.
-
A comprehensive map of copy number variations in dromedary camels based on whole genome sequence data.Sci Rep. 2024 Oct 26;14(1):25573. doi: 10.1038/s41598-024-77773-0. Sci Rep. 2024. PMID: 39462079 Free PMC article.
References
-
- Stice SL, Robl JM. Activation of mammalian oocytes by a factor obtained from rabbit sperm. Mol Reprod Dev 1990; 25:272–280. - PubMed
-
- Swann K. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 1990; 110:1295–1302. - PubMed
-
- Swann K, Lai FA. Egg activation at fertilization by a soluble sperm protein. Physiol Rev 2016; 96:127–149. - PubMed
-
- Hachem A, Godwin J, Ruas M, Lee HC, Ferrer BM, Ardestani G, Bassett A, Fox S, Navarrete F, de SP, Heindryckx B, Fissore R et al. . PLCzeta is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development 2017; 144:2914–2924. - PMC - PubMed
-
- Kimura Y, Yanagimachi R, Kuretake S, Bortkiewicz H, Perry AC, Yanagimachi H. Analysis of mouse oocyte activation suggests the involvement of sperm perinuclear material. Biol Reprod 1998; 58:1407–1415. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

