Circulating Testosterone as the Hormonal Basis of Sex Differences in Athletic Performance
- PMID: 30010735
- PMCID: PMC6391653
- DOI: 10.1210/er.2018-00020
Circulating Testosterone as the Hormonal Basis of Sex Differences in Athletic Performance
Abstract
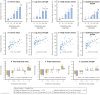
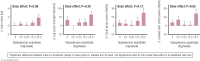
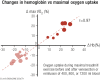
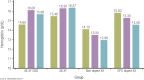
Elite athletic competitions have separate male and female events due to men's physical advantages in strength, speed, and endurance so that a protected female category with objective entry criteria is required. Prior to puberty, there is no sex difference in circulating testosterone concentrations or athletic performance, but from puberty onward a clear sex difference in athletic performance emerges as circulating testosterone concentrations rise in men because testes produce 30 times more testosterone than before puberty with circulating testosterone exceeding 15-fold that of women at any age. There is a wide sex difference in circulating testosterone concentrations and a reproducible dose-response relationship between circulating testosterone and muscle mass and strength as well as circulating hemoglobin in both men and women. These dichotomies largely account for the sex differences in muscle mass and strength and circulating hemoglobin levels that result in at least an 8% to 12% ergogenic advantage in men. Suppression of elevated circulating testosterone of hyperandrogenic athletes results in negative effects on performance, which are reversed when suppression ceases. Based on the nonoverlapping, bimodal distribution of circulating testosterone concentration (measured by liquid chromatography-mass spectrometry)-and making an allowance for women with mild hyperandrogenism, notably women with polycystic ovary syndrome (who are overrepresented in elite athletics)-the appropriate eligibility criterion for female athletic events should be a circulating testosterone of <5.0 nmol/L. This would include all women other than those with untreated hyperandrogenic disorders of sexual development and noncompliant male-to-female transgender as well as testosterone-treated female-to-male transgender or androgen dopers.
Figures



References
-
- Handelsman DJ. Performance enhancing hormones in sports doping In: DeGroot LJ, Jameson JL, eds. Endocrinology. 7th ed.Philadelphia, PA: Elsevier Saunders; 2015:441–454.
-
- Coleman DL. Sex in sport. Available at: ssrn.com/abstract=2928106. Accessed 22 October 2017.
-
- Lee PA, Nordenström A, Houk CP, Ahmed SF, Auchus R, Baratz A, Baratz Dalke K, Liao LM, Lin-Su K, Looijenga LH III, Mazur T, Meyer-Bahlburg HF, Mouriquand P, Quigley CA, Sandberg DE, Vilain E, Witchel S; Global DSD Update Consortium . Global disorders of sex development update since 2006: perceptions, approach and care [published correction appears in Horm Res Paediatr. 2016;85(3):180]. Horm Res Paediatr. 2016;85(3):158–180. - PubMed
-
- Southren AL, Tochimoto S, Carmody NC, Isurugi K. Plasma production rates of testosterone in normal adult men and women and in patients with the syndrome of feminizing testes. J Clin Endocrinol Metab. 1965;25(11):1441–1450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

