Development of Conjugate Addition of Lithium Dialkylcuprates to Thiochromones: Synthesis of 2-Alkylthiochroman-4-ones and Additional Synthetic Applications
- PMID: 30011953
- PMCID: PMC6099951
- DOI: 10.3390/molecules23071728
Development of Conjugate Addition of Lithium Dialkylcuprates to Thiochromones: Synthesis of 2-Alkylthiochroman-4-ones and Additional Synthetic Applications
Abstract
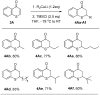
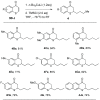
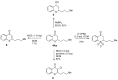
Lithium dialkylcuprates undergo conjugate addition to thiochromones to afford 2-alkylthiochroman-4-ones in good yields. This approach provide an efficient and general synthetic approach to privileged sulfur-containing structural motifs and valuable precursors for many pharmaceuticals, starting from common substrates-thiochromones. Good yields of 2-alkyl-substituted thiochroman-4-ones are attained with lithium dialkylcuprates, lithium alkylcyanocuprates or substoichiometric amount of copper salts. The use of commercially available inexpensive alkyllithium reagents will expedite the synthesis of a large library of 2-alkyl substituted thiochroman-4-ones for additional synthetic applications.
Keywords: 2-alkylthiochroman-4-ones; conjugate addition; lithium dialkyl cuprates; thiochroman-4-ones; thiochromones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

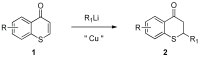
References
-
- Damani L.A., editor. Sulphur-Containing Drugs and Related Organic Compounds. Wiley; New York, NY, USA: 1989.
-
- Schneller S.W. Thiochromanones and Related Compounds. Adv. Heterocycl. Chem. 1975;18:59.
-
- Katritzky A.R., Rees C.W., editors. Comprehensive Heterocyclic Chemistry. Pergamon Press; Oxford, UK: 1984.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

