Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab
- PMID: 30012216
- PMCID: PMC6048712
- DOI: 10.1186/s40425-018-0383-1
Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab
Abstract
Background: Previous studies have suggested that elevated neutrophil-to-lymphocyte ratio (NLR) is prognostic for worse outcomes in patients with a variety of solid cancers, including those treated with immune checkpoint inhibitors.
Methods: This was a retrospective analysis of 97 consecutive patients with stage IV melanoma who were treated with nivolumab. Baseline NLR and derived (d) NLR were calculated and, along with other characteristics, correlated with progression-free survival (PFS) and overall survival (OS) in univariate and multivariate analyses. The best cutoff values for NLR and dNLR were derived using Cutoff Finder software based on an R routine which optimized the significance of the split between Kaplan-Meier survival curves.
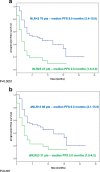
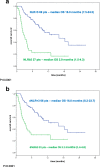
Results: In univariate analysis, increasing absolute neutrophil count (ANC), NLR, dNLR and lactate dehydrogenase (LDH) (continuous variables) were all significantly associated with OS. Only NLR (hazard ratio [HR] = 2.85; 95% CI 1.60-5.08; p < 0.0001) and LDH (HR = 2.51; 95% CI 1.36-4.64; p < 0.0001) maintained a significant association with OS in multivariate analysis. Patients with baseline NLR ≥5 had significantly worse OS and PFS than patients with NLR < 5, as did patients with baseline dNLR ≥3 versus < 3. Optimal cut-off values were ≥ 4.7 for NLR and ≥ 3.8 for dNLR. Using this ≥4.7 cut-off for NLR, the values for OS and PFS were overlapping to the canonical cut-off for values, and dNLR< 3.8 was also associated with better OS and PFS.
Conclusion: Both Neutrophil-to-lymphocyte ratio (NLR) and derived (d) NLR were associated with improved survival when baseline levels were lower than cut-off values. NLR and dNLR are simple, inexpensive and readily available biomarkers that could be used to help predict response to immunotherapy in patients with advanced melanoma.
Keywords: Biomarkers; Neutrophil-to-lymphocyte ratio; Nivolumab; PD-1 inhibitor.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Competing interests
P.A. Ascierto has/had a consultant/advisory role for Bristol Myers-Squibb, Roche-Genentech, Merck Sharp & Dohme, Array, Novartis, Amgen, Merck Serono, Pierre Fabre, Incyte, NewLink Genetics, Genmab, Medimmune. He also received Research funds from Bristol Myers-Squibb, Roche-Genentech, Array.
Antonio M. Grimaldi received honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis and Roche Genentech, and had consultant/advisory role for Merck Sharp & Dohme and Novartis.
E. Simeone participated to advisory board for Bristol-Myers Squibb and received honoraria from Bristol-Myers Squibb, Novartis and Merck Sharp & Dohme.
G. Palmieri has advisory role for Incyte, MSD, Novartis, Pierre-Fabre.
All the other authors have no conflict of interest to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
