Impact of the Number of Cycles of Platinum Based First Line Chemotherapy for Advanced Urothelial Carcinoma
- PMID: 30012366
- PMCID: PMC6814293
- DOI: 10.1016/j.juro.2018.07.035
Impact of the Number of Cycles of Platinum Based First Line Chemotherapy for Advanced Urothelial Carcinoma
Abstract
Purpose: We evaluated the impact of the number of cycles of platinum based, first line chemotherapy (fewer than 6 cycles vs the conventional 6 cycles or more) on the survival of patients with metastatic urothelial carcinoma.
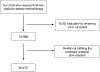
Materials and methods: We used the RISC (Retrospective International Study of Invasive/Advanced Cancer of the Urothelium) database. The association of the number of cycles of chemotherapy with overall survival was investigated by Cox multiple regression analysis after controlling for recognized prognostic factors. We excluded patients who received fewer than 3 or more than 9 platinum chemotherapy cycles to reduce confounding factors. The primary analysis was a comparison of overall survival for 3 to 5 vs 6 to 9 cycles using 6-month landmark analysis when 281 death events were observed.
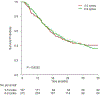
Results: Of the 1,020 patients in the RISC 472 received cisplatin or carboplatin, of whom 338 and 134, respectively, were evaluable. A total of 157 patients received 3 to 5 cycles (median 4) and 315 received 6 to 9 cycles (median 6). There was no significant difference in overall survival between 3 to 5 and 6 to 9 cycles (HR 1.02, 95% CI 0.78-1.33, p = 0.91). No significant interactions were observed for the type of platinum (p = 0.09) and completed planned chemotherapy (p = 0.56). The limitations of a hypothesis generating, retrospective analysis applied.
Conclusions: Four cycles of platinum based, first line chemotherapy appeared adequate and did not significantly compromise the survival of patients with advanced urothelial carcinoma. The omission of excessive cycles may avoid unnecessary cumulative toxicity and facilitate a better transition to second line therapy and investigational switch maintenance therapy strategies. These results require prospective validation but they may impact practice in select patients.
Keywords: carcinoma; cisplatin; mortality; urinary tract; urothelium.
Copyright © 2018 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Editorial Comment.J Urol. 2018 Dec;200(6):1214. doi: 10.1016/j.juro.2018.07.096. Epub 2018 Sep 4. J Urol. 2018. PMID: 30189185 No abstract available.
References
-
- von der Maase H, Sengelov L, Roberts JT et al. : Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol, 23: 4602, 2005 - PubMed
-
- Sternberg CN, de Mulder P, Schornagel JH et al. : Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer, 42: 50, 2006 - PubMed
-
- Saxman SB, Propert KJ, Einhorn LH et al. : Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol, 15: 2564, 1997 - PubMed
-
- Bellmunt J, von der Maase H, Mead GM et al. : Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol, 30: 1107, 2012 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

