Rickettsia Lipid A Biosynthesis Utilizes the Late Acyltransferase LpxJ for Secondary Fatty Acid Addition
- PMID: 30012728
- PMCID: PMC6148475
- DOI: 10.1128/JB.00334-18
Rickettsia Lipid A Biosynthesis Utilizes the Late Acyltransferase LpxJ for Secondary Fatty Acid Addition
Abstract
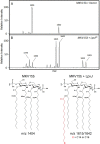
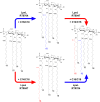
Members of the Rickettsia genus are obligate intracellular, Gram-negative coccobacilli that infect mammalian and arthropod hosts. Several rickettsial species are human pathogens and are transmitted by blood-feeding arthropods. In Gram-negative parasites, the outer membrane (OM) sits at the nexus of the host-pathogen interaction and is rich in lipopolysaccharide (LPS). The lipid A component of LPS anchors the molecule to the bacterial surface and is an endotoxic agonist of Toll-like receptor 4 (TLR4). Despite the apparent importance of lipid A in maintaining OM integrity, as well as its inflammatory potential during infection, this molecule is poorly characterized in Rickettsia pathogens. In this work, we have identified and characterized new members of the recently discovered LpxJ family of lipid A acyltransferases in both Rickettsia typhi and Rickettsia rickettsii, the etiological agents of murine typhus and Rocky Mountain spotted fever, respectively. Our results demonstrate that these enzymes catalyze the addition of a secondary acyl chain (C14/C16) to the 3'-linked primary acyl chain of the lipid A moiety in the final steps of the Raetz pathway of lipid A biosynthesis. Since lipid A architecture is fundamental to bacterial OM integrity, we believe that rickettsial LpxJ may be important in maintaining membrane dynamics to facilitate molecular interactions at the host-pathogen interface that are required for adhesion and invasion of mammalian cells. This work contributes to our understanding of rickettsial outer membrane physiology and sets a foundation for further exploration of the envelope and its role in pathogenesis.IMPORTANCE Lipopolysaccharide (LPS) triggers an inflammatory response through the TLR4-MD2 receptor complex and inflammatory caspases, a process mediated by the lipid A moiety of LPS. Species of Rickettsia directly engage both extracellular and intracellular immunosurveillance, yet little is known about rickettsial lipid A. Here, we demonstrate that the alternative lipid A acyltransferase, LpxJ, from Rickettsia typhi and R. rickettsii catalyzes the addition of C16 fatty acid chains into the lipid A 3'-linked primary acyl chain, accounting for major structural differences relative to the highly inflammatory lipid A of Escherichia coli.
Keywords: LPS; LpxJ; Rickettsia; lipid A; outer membrane; pathogenesis.
Copyright © 2018 Guillotte et al.
Figures




References
-
- Gillespie JJ, Nordberg EK, Azad AF, Sobral BWS. 2012. Phylogeny and comparative genomics: the shifting landscape in the genomics era, p 84–141. In Palmer GH, Azad AF (ed), Intracellular pathogens II: rickettsiales. ASM Press, Washington, DC.
-
- Winkler HH. 1986. Early events in the interaction of the obligate intracytoplasmic parasite, Rickettsia prowazekii, with eucaryotic cells: entry and lysis. Ann Inst Pasteur Microbiol 137A:333–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

