Effective Treatment of Mycobacterium avium subsp. hominissuis and Mycobacterium abscessus Species Infections in Macrophages, Biofilm, and Mice by Using Liposomal Ciprofloxacin
- PMID: 30012773
- PMCID: PMC6153787
- DOI: 10.1128/AAC.00440-18
Effective Treatment of Mycobacterium avium subsp. hominissuis and Mycobacterium abscessus Species Infections in Macrophages, Biofilm, and Mice by Using Liposomal Ciprofloxacin
Abstract
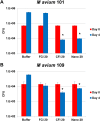
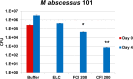
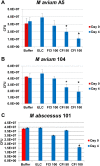
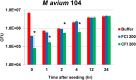
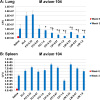
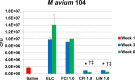
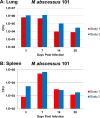
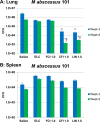
Nontuberculous mycobacteria (NTM) affect an increasing number of individuals worldwide. Infection with these organisms is more common in patients with chronic lung conditions, and treatment is challenging. Quinolones, such as ciprofloxacin, have been used to treat patients, but the results have not been encouraging. In this report, we evaluate novel formulations of liposome-encapsulated ciprofloxacin (liposomal ciprofloxacin) in vitro and in vivo Its efficacy against Mycobacterium avium and Mycobacterium abscessus was examined in macrophages, in biofilms, and in vivo using intranasal instillation mouse models. Liposomal ciprofloxacin was significantly more active than free ciprofloxacin against both pathogens in macrophages and biofilms. When evaluated in vivo, treatment with the liposomal ciprofloxacin formulations was associated with significant decreases in the bacterial loads in the lungs of animals infected with M. avium and M. abscessus In summary, topical delivery of liposomal ciprofloxacin in the lung at concentrations greater than those achieved in the serum can be effective in the treatment of NTM, and further evaluation is warranted.
Keywords: Mycobacterium abscessus; Mycobacterium avium; Mycobacterium avium subsp. hominissuis; biofilm; ciprofloxacin; infection; liposome; lung disease; mouse model; treatment.
Copyright © 2018 American Society for Microbiology.
Figures
Similar articles
-
In Vitro Efficacy of Free and Nanoparticle Formulations of Gallium(III) meso-Tetraphenylporphyrine against Mycobacterium avium and Mycobacterium abscessus and Gallium Biodistribution in Mice.Mol Pharm. 2018 Mar 5;15(3):1215-1225. doi: 10.1021/acs.molpharmaceut.7b01036. Epub 2018 Feb 16. Mol Pharm. 2018. PMID: 29421865 Free PMC article.
-
Sudapyridine (WX-081) antibacterial activity against Mycobacterium avium, Mycobacterium abscessus, and Mycobacterium chelonae in vitro and in vivo.mSphere. 2024 Feb 28;9(2):e0051823. doi: 10.1128/msphere.00518-23. Epub 2024 Jan 19. mSphere. 2024. PMID: 38240581 Free PMC article.
-
Nontuberculous Mycobacterial Lung Diseases Caused by Mixed Infection with Mycobacterium avium Complex and Mycobacterium abscessus Complex.Antimicrob Agents Chemother. 2018 Sep 24;62(10):e01105-18. doi: 10.1128/AAC.01105-18. Print 2018 Oct. Antimicrob Agents Chemother. 2018. PMID: 30104265 Free PMC article.
-
The double-edged sword of Tregs in M tuberculosis, M avium, and M absessus infection.Immunol Rev. 2021 May;301(1):48-61. doi: 10.1111/imr.12959. Epub 2021 Mar 12. Immunol Rev. 2021. PMID: 33713043 Free PMC article. Review.
-
Systematic Review and Meta-analyses of the Effect of Chemotherapy on Pulmonary Mycobacterium abscessus Outcomes and Disease Recurrence.Antimicrob Agents Chemother. 2017 Oct 24;61(11):e01206-17. doi: 10.1128/AAC.01206-17. Print 2017 Nov. Antimicrob Agents Chemother. 2017. PMID: 28807911 Free PMC article.
Cited by
-
Preclinical Models of Nontuberculous Mycobacteria Infection for Early Drug Discovery and Vaccine Research.Pathogens. 2020 Aug 6;9(8):641. doi: 10.3390/pathogens9080641. Pathogens. 2020. PMID: 32781698 Free PMC article. Review.
-
Mycobacterium abscessus subsp. massiliense: Biofilm Formation, Host Immune Response, and Therapeutic Strategies.Microorganisms. 2025 Feb 18;13(2):447. doi: 10.3390/microorganisms13020447. Microorganisms. 2025. PMID: 40005812 Free PMC article. Review.
-
Pipeline of anti-Mycobacterium abscessus small molecules: Repurposable drugs and promising novel chemical entities.Med Res Rev. 2021 Jul;41(4):2350-2387. doi: 10.1002/med.21798. Epub 2021 Mar 1. Med Res Rev. 2021. PMID: 33645845 Free PMC article. Review.
-
Nanobiosystems for Antimicrobial Drug-Resistant Infections.Nanomaterials (Basel). 2021 Apr 22;11(5):1075. doi: 10.3390/nano11051075. Nanomaterials (Basel). 2021. PMID: 33922004 Free PMC article. Review.
-
Inhaled Liposomal Antimicrobial Delivery in Lung Infections.Drugs. 2020 Sep;80(13):1309-1318. doi: 10.1007/s40265-020-01359-z. Drugs. 2020. PMID: 32691293 Free PMC article. Review.
References
-
- Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, Olivier KN. 2010. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 182:970–976. doi:10.1164/rccm.201002-0310OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

