The TMEM16A channel mediates the fast polyspermy block in Xenopus laevis
- PMID: 30012842
- PMCID: PMC6122928
- DOI: 10.1085/jgp.201812071
The TMEM16A channel mediates the fast polyspermy block in Xenopus laevis
Abstract
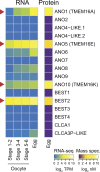
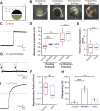
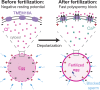
In externally fertilizing animals, such as sea urchins and frogs, prolonged depolarization of the egg immediately after fertilization inhibits the entry of additional sperm-a phenomenon known as the fast block to polyspermy. In the African clawed frog Xenopus laevis, this depolarization is driven by Ca2+-activated Cl- efflux. Although the prominent Ca2+-activated Cl- currents generated in immature X. laevis oocytes are mediated by X. laevis transmembrane protein 16a (xTMEM16A) channels, little is known about the channels that contribute to the fast block in mature eggs. Moreover, the gamete undergoes a gross transformation as it develops from an immature oocyte into a fertilization-competent egg. Here, we report the results of our approach to identify the Ca2+-activated Cl- channel that triggers the fast block. By querying published proteomic and RNA-sequencing data, we identify two Ca2+-activated Cl- channels expressed in fertilization-competent X. laevis eggs: xTMEM16A and X. laevis bestrophin 2A (xBEST2A). By exogenously expressing xTMEM16A and xBEST2A in axolotl cells lacking endogenous Ca2+-activated currents, we characterize the effect of inhibitors on currents mediated by these channels. None of the inhibitors tested block xBEST2A currents specifically. However, 2-(4-chloro-2-methylphenoxy)-N-[(2-methoxyphenyl)methylideneamino]-acetamide (Ani9) and N-((4-methoxy)-2-naphthyl)-5-nitroanthranilic acid (MONNA) each reduce xTMEM16A currents by more than 70% while only nominally inhibiting those generated by xBEST2A. Using whole-cell recordings during fertilization, we find that Ani9 and MONNA effectively diminish fertilization-evoked depolarizations. Additionally, these inhibitors lead to increased polyspermy in X. laevis embryos. These results indicate that fertilization activates TMEM16A channels in X. laevis eggs and induces the earliest known event triggered by fertilization: the fast block to polyspermy.
© 2018 Wozniak et al.
Figures

Comment in
-
The fast block to polyspermy: New insight into a century-old problem.J Gen Physiol. 2018 Sep 3;150(9):1233-1234. doi: 10.1085/jgp.201812145. Epub 2018 Aug 6. J Gen Physiol. 2018. PMID: 30082432 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

