Programmed Cell Death in the Pathogenesis of Influenza
- PMID: 30012970
- PMCID: PMC6073994
- DOI: 10.3390/ijms19072065
Programmed Cell Death in the Pathogenesis of Influenza
Abstract
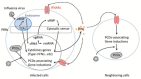
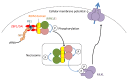
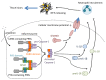
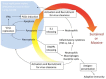
Influenza is a respiratory disease induced by infection by the influenza virus, which is a member of Orthomyxoviridae family. This infectious disease has serious impacts on public health systems and results in considerable mortality and economic costs throughout the world. Based on several experimental studies, massive host immune reaction is associated with the disease severity of influenza. Programmed cell death is typically induced during virus infection as a consequence of host immune reaction to limit virus spread by eliminating niches for virus propagation without causing inflammation. However, in some viral infectious diseases, such as influenza, in the process of immune reaction, aberrant induction of programmed cell death disturbs the maintenance of organ function. Current reports show that there are different types of programmed cell death that vary in terms of molecular mechanisms and/or associations with inflammation. In addition, these novel types of programmed cell death are associated with pathogenesis rather than suppressing virus propagation in the disease course. Here, we review our current understanding of mechanisms of programmed cell death in the pathogenesis of influenza.
Keywords: influenza; pathogenesis; programmed cell death.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

