Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota
- PMID: 30013066
- PMCID: PMC6048113
- DOI: 10.1038/s41467-018-05122-7
Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota
Abstract
By changing soil properties, plants can modify their growth environment. Although the soil microbiota is known to play a key role in the resulting plant-soil feedbacks, the proximal mechanisms underlying this phenomenon remain unknown. We found that benzoxazinoids, a class of defensive secondary metabolites that are released by roots of cereals such as wheat and maize, alter root-associated fungal and bacterial communities, decrease plant growth, increase jasmonate signaling and plant defenses, and suppress herbivore performance in the next plant generation. Complementation experiments demonstrate that the benzoxazinoid breakdown product 6-methoxy-benzoxazolin-2-one (MBOA), which accumulates in the soil during the conditioning phase, is both sufficient and necessary to trigger the observed phenotypic changes. Sterilization, fungal and bacterial profiling and complementation experiments reveal that MBOA acts indirectly by altering root-associated microbiota. Our results reveal a mechanism by which plants determine the composition of rhizosphere microbiota, plant performance and plant-herbivore interactions of the next generation.
Conflict of interest statement
The authors declare no competing interests.
Figures
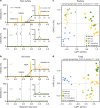
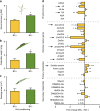
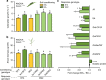
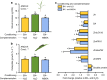
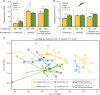
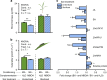
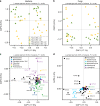
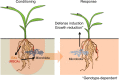
References
-
- van der Putten W, van Dijk C, Peters B. Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature. 1993;362:53. doi: 10.1038/362053a0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- 160786/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)/International
- 136184/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)/International
- 164480/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)/International
- 165891/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

