A diuranium carbide cluster stabilized inside a C80 fullerene cage
- PMID: 30013067
- PMCID: PMC6048043
- DOI: 10.1038/s41467-018-05210-8
A diuranium carbide cluster stabilized inside a C80 fullerene cage
Abstract
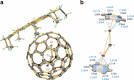
Unsupported non-bridged uranium-carbon double bonds have long been sought after in actinide chemistry as fundamental synthetic targets in the study of actinide-ligand multiple bonding. Here we report that, utilizing Ih(7)-C80 fullerenes as nanocontainers, a diuranium carbide cluster, U=C=U, has been encapsulated and stabilized in the form of UCU@Ih(7)-C80. This endohedral fullerene was prepared utilizing the Krätschmer-Huffman arc discharge method, and was then co-crystallized with nickel(II) octaethylporphyrin (NiII-OEP) to produce UCU@Ih(7)-C80·[NiII-OEP] as single crystals. X-ray diffraction analysis reveals a cage-stabilized, carbide-bridged, bent UCU cluster with unexpectedly short uranium-carbon distances (2.03 Å) indicative of covalent U=C double-bond character. The quantum-chemical results suggest that both U atoms in the UCU unit have formal oxidation state of +5. The structural features of UCU@Ih(7)-C80 and the covalent nature of the U(f1)=C double bonds were further affirmed through various spectroscopic and theoretical analyses.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Gregson M, Wooles AJ, Cooper OJ, Liddle ST. Covalent uranium carbene chemistry. Inorg. Chem. 2015;35:262–294.
-
- Patel D, Liddle ST. f-Element-metal bond chemistry. Rev. Inorg. Chem. 2012;32:1. doi: 10.1515/revic.2012.0001. - DOI
Publication types
Grants and funding
- 51772195/National Natural Science Foundation of China (National Science Foundation of China)/International
- 51772195/National Natural Science Foundation of China (National Science Foundation of China)/International
- 21433005/National Natural Science Foundation of China (National Science Foundation of China)/International
- DMR 1205302/National Science Foundation (NSF)/International
- DMR 1205302/National Science Foundation (NSF)/International
- CHE-1408865/National Science Foundation (NSF)/International
- AH-1922-20170325/Welch Foundation/International
- AH-1922-20170325/Welch Foundation/International
- BK20171211/Natural Science Foundation of Jiangsu Province (Jiangsu Provincial Natural Science Foundation)/International
- BK20171211/Natural Science Foundation of Jiangsu Province (Jiangsu Provincial Natural Science Foundation)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

