Structural basis for the regulation of inositol trisphosphate receptors by Ca2+ and IP3
- PMID: 30013099
- PMCID: PMC6082148
- DOI: 10.1038/s41594-018-0089-6
Structural basis for the regulation of inositol trisphosphate receptors by Ca2+ and IP3
Erratum in
-
Author Correction: Structural basis for the regulation of inositol trisphosphate receptors by Ca2+ and IP3.Nat Struct Mol Biol. 2018 Sep;25(9):902. doi: 10.1038/s41594-018-0119-4. Nat Struct Mol Biol. 2018. PMID: 30089818
Abstract
Inositol trisphosphate receptors (IP3Rs) are ubiquitous Ca2+-permeable channels that mediate release of Ca2+ from the endoplasmic reticulum, thereby regulating numerous processes including cell division, cell death, differentiation and fertilization. IP3Rs are jointly activated by inositol trisphosphate (IP3) and their permeant ion, Ca2+. At high concentrations, however, Ca2+ inhibits activity, ensuring precise spatiotemporal control over intracellular Ca2+. Despite extensive characterization of IP3R, the mechanisms through which these molecules control channel gating have remained elusive. Here, we present structures of full-length human type 3 IP3Rs in ligand-bound and ligand-free states. Multiple IP3-bound structures demonstrate that the large cytoplasmic domain provides a platform for propagation of long-range conformational changes to the ion-conduction gate. Structures in the presence of Ca2+ reveal two Ca2+-binding sites that induce the disruption of numerous interactions between subunits, thereby inhibiting IP3R. These structures thus provide a mechanistic basis for beginning to understand the regulation of IP3R.
Conflict of interest statement
The authors declare no competing interests.
Figures



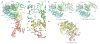
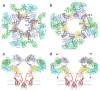


References
-
- Burgess GM, Mckinney JS, Fabiatoq A, Leslie BA, Putney JW. Calcium Pools in Saponin-permeabilized Guinea Pig Hepatocytes. J Biol Chem. 1983;258:35336–153345. - PubMed
-
- Ehrlich BE, Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988;336:583–586. - PubMed
-
- Furuichi T, et al. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. - PubMed
-
- Mignery GA, Südhof TC, Takei K, De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–5. - PubMed
-
- Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

