Structural determinants of specificity and regulation of activity in the allosteric loop network of human KLK8/neuropsin
- PMID: 30013126
- PMCID: PMC6048020
- DOI: 10.1038/s41598-018-29058-6
Structural determinants of specificity and regulation of activity in the allosteric loop network of human KLK8/neuropsin
Abstract
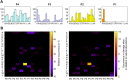
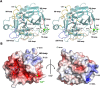
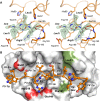
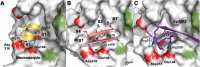
Human KLK8/neuropsin, a kallikrein-related serine peptidase, is mostly expressed in skin and the hippocampus regions of the brain, where it regulates memory formation by synaptic remodeling. Substrate profiles of recombinant KLK8 were analyzed with positional scanning using fluorogenic tetrapeptides and the proteomic PICS approach, which revealed the prime side specificity. Enzyme kinetics with optimized substrates showed stimulation by Ca2+ and inhibition by Zn2+, which are physiological regulators. Crystal structures of KLK8 with a ligand-free active site and with the inhibitor leupeptin explain the subsite specificity and display Ca2+ bound to the 75-loop. The variants D70K and H99A confirmed the antagonistic role of the cation binding sites. Molecular docking and dynamics calculations provided insights in substrate binding and the dual regulation of activity by Ca2+ and Zn2+, which are important in neuron and skin physiology. Both cations participate in the allosteric surface loop network present in related serine proteases. A comparison of the positional scanning data with substrates from brain suggests an adaptive recognition by KLK8, based on the tertiary structures of its targets. These combined findings provide a comprehensive picture of the molecular mechanisms underlying the enzyme activity of KLK8.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- P25003-B21/Austrian Science Fund (FWF Der Wissenschaftsfonds)/International
- P41 CA196276/CA/NCI NIH HHS/United States
- Graduiertenkolleg 333/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- I631-B11/Austrian Science Fund (FWF Der Wissenschaftsfonds)/International
- T32 GM064337/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

