Fetal gene therapy for neurodegenerative disease of infants
- PMID: 30013199
- PMCID: PMC6130799
- DOI: 10.1038/s41591-018-0106-7
Fetal gene therapy for neurodegenerative disease of infants
Abstract
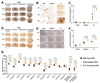
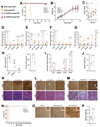
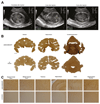
For inherited genetic diseases, fetal gene therapy offers the potential of prophylaxis against early, irreversible and lethal pathological change. To explore this, we studied neuronopathic Gaucher disease (nGD), caused by mutations in GBA. In adult patients, the milder form presents with hepatomegaly, splenomegaly and occasional lung and bone disease; this is managed, symptomatically, by enzyme replacement therapy. The acute childhood lethal form of nGD is untreatable since enzyme cannot cross the blood-brain barrier. Patients with nGD exhibit signs consistent with hindbrain neurodegeneration, including neck hyperextension, strabismus and, often, fatal apnea1. We selected a mouse model of nGD carrying a loxP-flanked neomycin disruption of Gba plus Cre recombinase regulated by the keratinocyte-specific K14 promoter. Exclusive skin expression of Gba prevents fatal neonatal dehydration. Instead, mice develop fatal neurodegeneration within 15 days2. Using this model, fetal intracranial injection of adeno-associated virus (AAV) vector reconstituted neuronal glucocerebrosidase expression. Mice lived for up to at least 18 weeks, were fertile and fully mobile. Neurodegeneration was abolished and neuroinflammation ameliorated. Neonatal intervention also rescued mice but less effectively. As the next step to clinical translation, we also demonstrated the feasibility of ultrasound-guided global AAV gene transfer to fetal macaque brains.
Conflict of interest statement
Seng H. Cheng is an employee at Sanofi, a biopharmaceutical company
Figures

Comment in
-
Gene therapy in mouse fetuses treats deadly disease.Nature. 2018 Jul;559(7714):313-314. doi: 10.1038/d41586-018-05726-5. Nature. 2018. PMID: 30018442 No abstract available.
-
Fetal gene therapy could be feasible for neuronopathic Gaucher disease.Nat Rev Neurol. 2018 Sep;14(9):507. doi: 10.1038/s41582-018-0053-4. Nat Rev Neurol. 2018. PMID: 30061714 No abstract available.
-
Delivering gene therapy.Nat Rev Neurosci. 2018 Sep;19(9):515. doi: 10.1038/s41583-018-0051-y. Nat Rev Neurosci. 2018. PMID: 30087446 No abstract available.
-
Future AAVenues for In Utero Gene Therapy.Cell Stem Cell. 2018 Sep 6;23(3):320-321. doi: 10.1016/j.stem.2018.08.010. Cell Stem Cell. 2018. PMID: 30193130
References
-
- Farfel-Becker T, et al. Spatial and temporal correlation between neuron loss and neuroinflammation in a mouse model of neuronopathic Gaucher disease. Hum Mol Genet. 2011 - PubMed
Publication types
MeSH terms
Grants and funding
- MR/R015325/1/MRC_/Medical Research Council/United Kingdom
- MR/R025134/1/MRC_/Medical Research Council/United Kingdom
- MR/N019075/1/MRC_/Medical Research Council/United Kingdom
- G1000709/MRC_/Medical Research Council/United Kingdom
- MR/M00676X/1/MRC_/Medical Research Council/United Kingdom
- 202834/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- 260862/ERC_/European Research Council/International
- MR/N026101/1/MRC_/Medical Research Council/United Kingdom
- NC/L001780/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- MR/P026494/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

