Intravital imaging-based analysis tools for vessel identification and assessment of concurrent dynamic vascular events
- PMID: 30013228
- PMCID: PMC6048163
- DOI: 10.1038/s41467-018-04929-8
Intravital imaging-based analysis tools for vessel identification and assessment of concurrent dynamic vascular events
Abstract
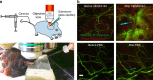
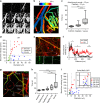
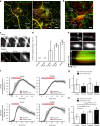
The vasculature undergoes changes in diameter, permeability and blood flow in response to specific stimuli. The dynamics and interdependence of these responses in different vessels are largely unknown. Here we report a non-invasive technique to study dynamic events in different vessel categories by multi-photon microscopy and an image analysis tool, RVDM (relative velocity, direction, and morphology) allowing the identification of vessel categories by their red blood cell (RBC) parameters. Moreover, Claudin5 promoter-driven green fluorescent protein (GFP) expression is used to distinguish capillary subtypes. Intradermal injection of vascular endothelial growth factor A (VEGFA) is shown to induce leakage of circulating dextran, with vessel-type-dependent kinetics, from capillaries and venules devoid of GFP expression. VEGFA-induced leakage in capillaries coincides with vessel dilation and reduced flow velocity. Thus, intravital imaging of non-invasive stimulation combined with RVDM analysis allows for recording and quantification of very rapid events in the vasculature.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- 2015-02375_3/Vetenskapsrådet (Swedish Research Council)/International
- 16 0585/Cancerfonden (Swedish Cancer Society)/International
- 2015.0275/Knut och Alice Wallenbergs Stiftelse (Knut and Alice Wallenberg Foundation)/International
- KAW 2015.0030/Knut och Alice Wallenbergs Stiftelse (Knut and Alice Wallenberg Foundation)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

