Overuse of inhaled corticosteroids in COPD: five questions for withdrawal in daily practice
- PMID: 30013336
- PMCID: PMC6039066
- DOI: 10.2147/COPD.S164259
Overuse of inhaled corticosteroids in COPD: five questions for withdrawal in daily practice
Abstract
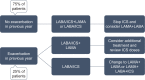
Evidence and guidelines are becoming increasingly clear about imbalance between the risks and benefits of inhaled corticosteroids (ICSs) in patients with COPD. While selected patients may benefit from ICS-containing regimens, ICSs are often inappropriately prescribed with - according to Belgian market research data - up to 70% of patients in current practice receiving ICSs, usually as a fixed combination with a long-acting β2-adrenoreceptor agonist. Studies and recommendations support withdrawal of ICSs in a large group of patients with COPD. However, historical habits appear difficult to change even in the light of recent scientific evidence. We have built a collaborative educational platform with chest physicians and primary care physicians to increase awareness and provide guidance and support in this matter.
Keywords: COPD; education; exacerbation; inhaled steroids; systematic review; withdrawal.
Conflict of interest statement
Disclosure DC is the founder of Aquilon Pharmaceuticals and had received speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, Mundipharma, Chiesi, and GlaxoSmithKline (GSK) and consultancy fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Novartis for the participation to advisory boards. ED’s clinical department received financial support from Boehringer Ingelheim and Novartis to perform clinical studies. He participated in advisory boards by Boehringer Ingelheim, Chiesi, Cipla, Novartis, and AstraZeneca, for which a fee was given to the clinical department. He received travel grants from Boehringer Ingelheim, GSK, and AstraZeneca to attend international congresses. He also received speaker fees from Boehringer Ingelheim, GSK, AstraZeneca, and Novartis to give scientific presentations to local GP groupings for which a fee was given to the clinical department. GL received speaker fees from Boehringer Ingelheim, Novartis, and Chiesi and consultancy fees from AstraZeneca, Boehringer Ingelheim, Chiesi, and Novartis for the participation to advisory boards. EM received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, and Novartis and consultancy fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, and Novartis for the participation to advisory boards. VN received speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, and Bristol-Myers Squibb and consultancy fees from AstraZeneca, Boehringer Ingelheim, Novartis, Bristol-Myers Squibb, and GSK for the participation to advisory boards. RP received speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, and GSK and consultancy fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, and Novartis for the participation to advisory boards. HS received speaker fees from AstraZeneca, Boehringer Ingelheim, and Roche and consultancy fees from Boehringer Ingelheim, GSK, and Roche for the participation to advisory boards. WV has been a member of advisory boards of and/or has been a speaker for AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, and Novartis. WJ is a senior clinical researcher of the FWO. He is a chair holder of the Belgian AstraZeneca Chair for Respiratory Pathophysiology. He received research grants and consultancy fees from Boehringer Ingelheim, AstraZeneca, Novartis, Chiesi, and GSK. The authors report no other conflicts of interest in this work.
Figures

References
-
- Jochmann A, Neubauer F, Miedinger D, Schafroth S, Tamm M, Leuppi JD. General practitioner’s adherence to the COPD GOLD guidelines: baseline data of the Swiss COPD Cohort Study. Swiss Med Wkly. Epub 2010 Apr 21. - PubMed
-
- Lucas AE, Smeenk FW, Smeele IJ, van Schayck CP. Overtreatment with inhaled corticosteroids and diagnostic problems in primary care patients, an exploratory study. Fam Pract. 2008;25(2):86–91. - PubMed
-
- Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

