By blocking hexokinase-2 phosphorylation, limonin suppresses tumor glycolysis and induces cell apoptosis in hepatocellular carcinoma
- PMID: 30013360
- PMCID: PMC6037266
- DOI: 10.2147/OTT.S165220
By blocking hexokinase-2 phosphorylation, limonin suppresses tumor glycolysis and induces cell apoptosis in hepatocellular carcinoma
Abstract
Introduction: The purpose of present study was to investigate the effect of limonin on tumor glycolysis and the underlying mechanisms in hepatocellular carcinoma (HCC).
Methods: Cell proliferation and colony formation assays were performed to evaluate the potency of limonin against HCC cells in vitro. The glucose consumption and lactate production after limonin treatment was determined. The effect of limonin on hexokinase-2 (HK-2) activity was assessed and the mitochondrial location of HK-2 was studied by immunoprecipitation. Cell apoptosis and protein expression were detected by flow cytometry and western blotting respectively. Protein overexpression by plasmid transfection was adopted to investigate the molecular mechanisms.
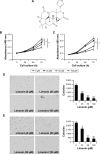
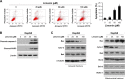
Results: HCC proliferation and colony formation were inhibited by limonin in vitro. With the suppression of HK-2 activity, the glycolytic level in HCC cells was substantially reduced, which was evidenced by the decrease of glucose consumption and lactate production. The phosphorylation of HK-2 was substantially inhibited by limonin, which resulted in the disassociation of HK-2 from mitochondria. Due to the reduction of HK-2 in mitochondria, increasing Bax were shifted to the mitochondria and gave rise to the release of cytochrome C, which induced HCC cells to subject to mitochondria-mediated apoptosis. Mechanism investigations revealed that the decrease of HK-2 phosphorylation was mainly due to the inhibition of Akt activity. In Akt exogenously overexpressed HCC cells, limonin-mediated cell proliferation inhibition, glycolysis suppression and apoptosis induction were significantly impaired.
Conclusion: Limonin inhibited the tumor glycolysis in hepatocellular carcinoma by suppressing HK-2 activity, and the suppression of HK-2 was closely related to the decrease of Akt activity.
Keywords: apoptosis; hexokinase-2; limonin; tumor glycolysis.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2.J Exp Clin Cancer Res. 2017 Mar 20;36(1):44. doi: 10.1186/s13046-017-0514-4. J Exp Clin Cancer Res. 2017. PMID: 28320429 Free PMC article.
-
Butein Inhibited In Vitro Hexokinase-2-Mediated Tumor Glycolysis in Hepatocellular Carcinoma by Blocking Epidermal Growth Factor Receptor (EGFR).Med Sci Monit. 2018 May 19;24:3283-3292. doi: 10.12659/MSM.906528. Med Sci Monit. 2018. PMID: 29777095 Free PMC article.
-
Penfluridol triggers mitochondrial-mediated apoptosis and suppresses glycolysis in colorectal cancer cells through down-regulating hexokinase-2.Anat Rec (Hoboken). 2021 Mar;304(3):520-530. doi: 10.1002/ar.24464. Epub 2020 Jul 12. Anat Rec (Hoboken). 2021. Retraction in: Anat Rec (Hoboken). 2021 Dec;304(12):2893. doi: 10.1002/ar.24805. PMID: 32470200 Retracted.
-
Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy.Cell Death Differ. 2015 Feb;22(2):248-57. doi: 10.1038/cdd.2014.173. Epub 2014 Oct 17. Cell Death Differ. 2015. PMID: 25323588 Free PMC article. Review.
-
The Promoting Role of HK II in Tumor Development and the Research Progress of Its Inhibitors.Molecules. 2023 Dec 22;29(1):75. doi: 10.3390/molecules29010075. Molecules. 2023. PMID: 38202657 Free PMC article. Review.
Cited by
-
Metabolomic Analysis of Phytochemical Compounds from Ethanolic Extract of Lime (Citrus aurantifolia) Peel and Its Anti-Cancer Effects against Human Hepatocellular Carcinoma Cells.Molecules. 2023 Mar 26;28(7):2965. doi: 10.3390/molecules28072965. Molecules. 2023. PMID: 37049726 Free PMC article.
-
Dioscin Inhibited Glycolysis and Induced Cell Apoptosis in Colorectal Cancer via Promoting c-myc Ubiquitination and Subsequent Hexokinase-2 Suppression.Onco Targets Ther. 2020 Jan 6;13:31-44. doi: 10.2147/OTT.S224062. eCollection 2020. Onco Targets Ther. 2020. PMID: 32021252 Free PMC article.
-
Role of limonin in anticancer effects of Evodia rutaecarpa on ovarian cancer cells.BMC Complement Med Ther. 2020 Mar 20;20(1):94. doi: 10.1186/s12906-020-02890-y. BMC Complement Med Ther. 2020. PMID: 32197606 Free PMC article.
-
Natural products targeting glycolytic signaling pathways-an updated review on anti-cancer therapy.Front Pharmacol. 2022 Oct 20;13:1035882. doi: 10.3389/fphar.2022.1035882. eCollection 2022. Front Pharmacol. 2022. PMID: 36339566 Free PMC article. Review.
-
Natural compounds as lactate dehydrogenase inhibitors: potential therapeutics for lactate dehydrogenase inhibitors-related diseases.Front Pharmacol. 2023 Oct 17;14:1275000. doi: 10.3389/fphar.2023.1275000. eCollection 2023. Front Pharmacol. 2023. PMID: 37915411 Free PMC article. Review.
References
-
- Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292(5516):504–507. - PubMed
-
- Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206(Pt 12):2049–2057. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

