Assessing the response of morphea and limited scleroderma to tranilast: a small prospective study comparing topical corticosteroids to a combination of topical corticosteroids and tranilast
- PMID: 30013378
- PMCID: PMC6037270
- DOI: 10.2147/CCID.S160923
Assessing the response of morphea and limited scleroderma to tranilast: a small prospective study comparing topical corticosteroids to a combination of topical corticosteroids and tranilast
Abstract
Background: Scleroderma is traditionally managed with immunomodulatory agents such as methotrexate, mycophenolate mofetil and corticosteroids. There are anecdotal reports for, and theoretical reasons why, the anti-fibrotic agent tranilast may provide an additional treatment modality.
Objective: The objective of the current study was to demonstrate if the addition of topical tranilast to an established regime resulted in an improvement in the Localized Scleroderma Assessment Tool (LoSCAT) and modified Rodnan score.
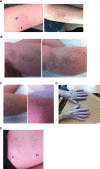
Patients and methods: A small double-blinded randomized prospective study of 11 pairs of treatment sites in four patients; three with morphea and one with limited scleroderma was performed. All patients continued with their prescribed treatment and applied 0.1% betamethasone valerate in PCCA PracaSil™ (B) to the control site with 0.1% betamethasone valerate and 1% tranilast (B/T) to the comparator site over a period of 3 months. Photographs and monthly LoSCAT scores were performed on the morphea patients and a modified Rodnan score on the limited scleroderma patient. Statistical analysis was via sign test.
Results: The mean baseline LoScat score at the B treated sites was 6.6 which improved to 4.3 (p= 0.16). The mean baseline LoScat score at the B/T treated sites was 5.75 which improved to 2.8 following treatment. (p=0.04).
Limitations: This was a small single center study. The ideal concentration of tranilast is unknown. As all patients continued with standard management the expected response may be less than would have been anticipated in a single agent trial.
Conclusion: The role of tranilast in the management in scleroderma warrants further investigation in larger trials.
Keywords: kynurenine; limited scleroderma; morphea; tranilast.
Conflict of interest statement
Disclosure The author has no conflicts of interest in this work.
Figures
References
-
- Peterson LS, Nelson AM, Su WP. Classification of morphea (localized scleroderma) Mayo Clin Proc. 1995;70:1068–1076. - PubMed
-
- Fett N, Werth VP. Update on morphea: part I. Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64(2):217–228. - PubMed
-
- Kräling BM, Maul GG, Jimenez SA. Mononuclear cellular infiltrates in clinically involved skin from patients with systemic sclerosis of recent onset predominantly consist of monocytes/macrophages. Pathobiology. 1995;63(1):48–56. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

