Recent advances in antiemetics: new formulations of 5HT3-receptor antagonists
- PMID: 30013391
- PMCID: PMC6037149
- DOI: 10.2147/CMAR.S166912
Recent advances in antiemetics: new formulations of 5HT3-receptor antagonists
Abstract
Purpose: To discuss new therapeutic strategies for chemotherapy-induced nausea and vomiting (CINV) involving 5-hydroxytryptamine type 3 (5HT3)-receptor antagonists (RAs).
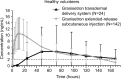
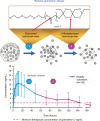
Summary: CINV remains poorly controlled in patients receiving moderately emetogenic chemotherapy (MEC) or highly emetogenic chemotherapy (HEC); nausea and delayed-phase CINV (24-120 hours after chemotherapy) are the most difficult to control. National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) antiemesis-guideline recommendations for HEC include a four-drug regimen (5HT3 RA, neurokinin 1 [NK1] RA, dexamethasone, and olanzapine). For some MEC regimens, a three-drug regimen (5HT3 RA, NK1 RA, and dexamethasone) is recommended. While 5HT3 RAs have dramatically improved CINV in the acute phase (0-24 hours after chemotherapy), their efficacy declines in the delayed phase. Newer formulations have been developed to extend 5HT3-RA efficacy into the delayed phase. Granisetron extended-release subcutaneous (GERSC), the most recently approved 5HT3 RA, provides slow, controlled release of therapeutic granisetron concentrations for ≥5 days. GERSC is included in the NCCN and ASCO guidelines for MEC and HEC, with NCCN-preferred status for MEC in the absence of an NK1 RA. Efficacy and safety of 5HT3 RAs in the context of guideline-recommended antiemetic therapy are reviewed.
Conclusion: Recent updates in antiemetic guidelines and the development of newer antiemet-ics should help mitigate CINV, this dreaded side effect of chemotherapy. GERSC, the most recently approved 5HT3-RA formulation, is indicated for use with other antiemetics to prevent acute and delayed nausea and vomiting associated with initial and repeat courses of MEC and anthracycline-cyclophosphamide combination-chemotherapy regimens.
Keywords: chemotherapy-induced nausea and vomiting; granisetron; granisetron extended-release injection; granisetron extended-release subcutaneous; serotonin-receptor antagonist.
Conflict of interest statement
Disclosure JG has served as a member of a speaker bureau or advisory committee for Heron Therapeutics. LS has acted in a consultant/advisory role for Eisai, Helsinn, Merck, and Tesaro, received research funding from Helsinn, and served as a member of a speaker bureau or advisory committee for Helsinn and Merck. NG has served as a member of a speaker bureau or advisory committee for Eisai, Heron, and Tesaro. The authors report no other conflicts of interest in this work.
Figures
References
-
- Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24(27):4472–4478. - PubMed
-
- Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73(3):249–262. - PubMed
-
- Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15(5):497–503. - PubMed
-
- Haiderali A, Menditto L, Good M, Teitelbaum A, Wegner J. Impact on daily functioning and indirect/direct costs associated with chemotherapy-induced nausea and vomiting (CINV) in a U.S. population. Support Care Cancer. 2011;19(6):843–851. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

