Safety and efficacy of denosumab in the treatment of pulmonary metastatic giant cell tumor of bone
- PMID: 30013396
- PMCID: PMC6038885
- DOI: 10.2147/CMAR.S161871
Safety and efficacy of denosumab in the treatment of pulmonary metastatic giant cell tumor of bone
Abstract
Background: Giant cell tumor (GCT) of bone is an intermittent and locally aggressive tumor with increasing pulmonary metastatic potential. In this study, we evaluated the interim clinical outcome of denosumab in patients with pulmonary metastatic GCT.
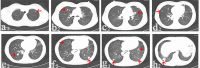
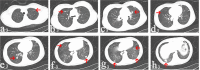
Materials and methods: We retrospectively reviewed seven patients with pulmonary metastatic GCT who received denosumab treatment after local tumor surgery during January 2014 and July 2016. Denosumab treatment for all patients lasted for at least 12 months. Serial chest computerized tomography scan was used to monitor the drug response and RECIST 1.1 standard was used to evaluate the therapeutic efficacy.
Results: All patients experienced chest pain relief in the first month of treatment. Three patients showed partial response. Four patients got stable disease after denosumab treatment. Adverse events included one patient with hypocalcemia and two patients with fever. No treatment-related deaths were reported. No patient with metastatic disease progression was found during an average of 28.6 months follow-up period.
Conclusion: We presented a promising interim clinical outcome using denosumab to treat patients with pulmonary metastatic GCT. Denosumab might be considered as the first-line treatment for patients with inoperable metastatic pulmonary GCT. However, Phase II clinical study with larger number of patients and longer follow-up period is needed to detect the further efficacy and safety of this drug for lung metastatic GCT.
Keywords: RANKL; denosumab; giant cell tumor of bone; pulmonary metastasis; recurrence.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
-
- Mendenhall WM, Zlotecki RA, Scarborough MT, Gibbs CP, Mendenhall NP. Giant cell tumor of bone. Am J Clin Oncol. 2006;29(1):96–99. - PubMed
-
- Niu X, Zhang Q, Hao L, et al. Giant cell tumor of the extremity: retrospective analysis of 621 Chinese patients from one institution. J Bone Joint Surg Am. 2012;94(5):461–467. - PubMed
-
- Bertoni F, Present D, Sudanese A, Baldini N, Bacchini P, Campanacci M. Giant-cell tumor of bone with pulmonary metastases. Six case reports and a review of the literature. Clin Orthop Relat Res. 1988;237:275–285. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

