Insights Into Mucosal-Associated Invariant T Cell Biology From Studies of Invariant Natural Killer T Cells
- PMID: 30013556
- PMCID: PMC6036249
- DOI: 10.3389/fimmu.2018.01478
Insights Into Mucosal-Associated Invariant T Cell Biology From Studies of Invariant Natural Killer T Cells
Abstract
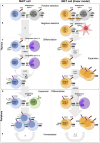
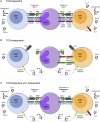
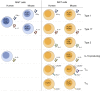
Mucosal-associated invariant T (MAIT) cells and invariant natural killer T (iNKT) cells are innate-like T cells that function at the interface between innate and adaptive immunity. They express semi-invariant T cell receptors (TCRs) and recognize unconventional non-peptide ligands bound to the MHC Class I-like molecules MR1 and CD1d, respectively. MAIT cells and iNKT cells exhibit an effector-memory phenotype and are enriched within the liver and at mucosal sites. In humans, MAIT cell frequencies dwarf those of iNKT cells, while in laboratory mouse strains the opposite is true. Upon activation via TCR- or cytokine-dependent pathways, MAIT cells and iNKT cells rapidly produce cytokines and show direct cytotoxic activity. Consequently, they are essential for effective immunity, and alterations in their frequency and function are associated with numerous infectious, inflammatory, and malignant diseases. Due to their abundance in mice and the earlier development of reagents, iNKT cells have been more extensively studied than MAIT cells. This has led to the routine use of iNKT cells as a reference population for the study of MAIT cells, and such an approach has proven very fruitful. However, MAIT cells and iNKT cells show important phenotypic, functional, and developmental differences that are often overlooked. With the recent availability of new tools, most importantly MR1 tetramers, it is now possible to directly study MAIT cells to understand their biology. Therefore, it is timely to compare the phenotype, development, and function of MAIT cells and iNKT cells. In this review, we highlight key areas where MAIT cells show similarity or difference to iNKT cells. In addition, we discuss important avenues for future research within the MAIT cell field, especially where comparison to iNKT cells has proven less informative.
Keywords: activation; development; effector function; innate-like T cells; mucosal-associated invariant T cells; natural killer T cells; phenotype; subsets.
Figures
References
-
- Hill TM, Bezbradica JS, Van Kaer L, Joyce S. CD1d-restricted natural killer T cells. In: eLS. Chichester: John Wiley & Sons, Ltd; (2016).10.1002/9780470015902.a0020180.pub2 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

