Evaluation of the Protective Effect of Olive Leaf Extract on Cisplatin-Induced Testicular Damage in Rats
- PMID: 30013722
- PMCID: PMC6022323
- DOI: 10.1155/2018/8487248
Evaluation of the Protective Effect of Olive Leaf Extract on Cisplatin-Induced Testicular Damage in Rats
Abstract
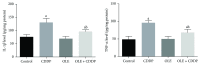
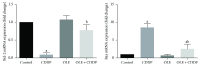
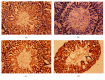
In the present investigation, the effect of olive leaf extract (OLE) on testicular damage induced in rats by an intraperitoneal injection of cisplatin (cis-diamminedichloroplatinum (CDDP)) at a dose of 5 mg/kg was tested. Rats were randomly divided into 4 groups: control, CDDP, OLE, and OLE + CDDP. After 5 days of CDDP treatment, body and testicular weights, histopathological alteration, and serum male sex hormone levels were determined. In addition to the biochemical and immunohistochemical changes in the testes, CDDP caused the disorganization of germinal epithelium and apoptosis by inducing Bax and inhibiting Bcl-2 protein expression. Testicular weights, catalase, serum testosterone, testicular enzymatic (including glutathione peroxidase, glutathione reductase, and superoxide dismutase) along with nonenzymatic (glutathione) antioxidants, and levels of luteinizing and follicle-stimulating hormones were significantly reduced in addition to a significant increase in testicular malondialdehyde and nitrite/nitrate levels when compared with the control group. OLE treatment markedly attenuated both biochemical and histopathological changes. The reproductive beneficial effects of OLE were mediated, at least partly, by inducing the nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase 1 (HO-1) pathway.
Figures

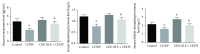
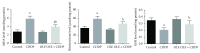





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

