Association of MOG-IgG Serostatus With Relapse After Acute Disseminated Encephalomyelitis and Proposed Diagnostic Criteria for MOG-IgG-Associated Disorders
- PMID: 30014148
- PMCID: PMC6248120
- DOI: 10.1001/jamaneurol.2018.1814
Association of MOG-IgG Serostatus With Relapse After Acute Disseminated Encephalomyelitis and Proposed Diagnostic Criteria for MOG-IgG-Associated Disorders
Abstract
Importance: Recent studies have reported a higher relapse rate following an initial inflammatory demyelinating disorder in pediatric patients with persistent seropositivity of antibodies targeting myelin oligodendrocyte glycoprotein (MOG-IgG1). To date, the clinical implications of longitudinal MOG-IgG1 seropositivity using live cell assays with IgG1 secondary antibodies in adults after acute disseminated encephalomyelitis (ADEM) are unknown.
Objective: To determine whether MOG-IgG1 serostatus (transient vs persistent) and titer change over time provide clinical utility in predicting the likelihood of relapse after ADEM.
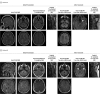
Design, setting, and participants: This cohort study identified patients with an initial diagnosis of ADEM evaluated at a single referral center between January 1, 1990, and October 1, 2017. Fifty-one patients were included, including 31 children and 20 adults. Longitudinal serologic testing was performed detecting autoantibodies targeting aquaporin 4 (AQP4-IgG) and MOG-IgG1 with clinically validated fluorescence-activated cell sorting assays. Patients were divided into 3 cohorts: persistent seropositivity, transient seropositivity, and seronegativity.
Main outcomes and measures: Clinical demographic characteristics, longitudinal AQP4-IgG and MOG-IgG1 serostatus, titers, relapses, use of immunotherapy, and Expanded Disability Status Scale score at follow-up.
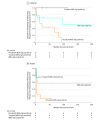
Results: Of 51 patients presenting with an initial diagnosis of ADEM, 20 (39%) were adult, 24 (47%) were female, and ages ranged from 12 months to 57 years. Seventeen patients fulfilled criteria for persistent seropositivity; of those, 8 of 9 children (89%) and 7 of 8 adults (88%) had at least 1 relapse after median (range) follow-up periods of 75 (15-236) months and 39 (9-161) months, respectively. Eight patients (16%), including 4 adults, fulfilled criteria for transient seropositivity; of those, no children and 1 of 4 adults (25%) relapsed after median (range) follow-up periods of 32 (24-114) months and 16 (13-27) months, respectively. Of 24 patients with AQP4-IgG and MOG-IgG seronegativity, 6 of 17 children (35%) and 2 of 7 adults (29%) had at least 1 relapse after median (range) follow-up periods of 36 (3-203) months and 34 (15-217) months, respectively. There were only 2 patients, including 1 adult, with AQP4-IgG seropositivity, and both relapsed. The hazard ratio for relapses in those with persistent MOG-IgG1 positivity compared with AQP4-IgG and MOG-IgG1 seronegativity was 3.1 (95% CI, 1.1-8.9; P = .04) in children and 5.5 (95% CI, 1.4-22.5; P = .02) in adults. Immunotherapy was used in 5 of 9 children (56%) and 6 of 8 adults (75%) with persistent seropositivity and in 3 of 17 children (18%) and 1 of 7 adults (14%) with AQP4-IgG and MOG-IgG seronegativity.
Conclusions and relevance: Relapse occurred in 15 of 17 patients (88%) with persistent MOG-IgG1 seropositivity after ADEM; only 1 patient with transient seropositivity experienced relapse. Our data extend the clinical utility of MOG-IgG1 serological testing to adult patients and highlights that longitudinal serologic evaluation of MOG-IgG1 could help predict disease course and consideration of immunotherapy.
Conflict of interest statement
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

