Subcellular origin of mitochondrial DNA deletions in human skeletal muscle
- PMID: 30014514
- PMCID: PMC6141001
- DOI: 10.1002/ana.25288
Subcellular origin of mitochondrial DNA deletions in human skeletal muscle
Abstract
Objective: In patients with mitochondrial DNA (mtDNA) maintenance disorders and with aging, mtDNA deletions sporadically form and clonally expand within individual muscle fibers, causing respiratory chain deficiency. This study aimed to identify the sub-cellular origin and potential mechanisms underlying this process.
Methods: Serial skeletal muscle cryosections from patients with multiple mtDNA deletions were subjected to subcellular immunofluorescent, histochemical, and genetic analysis.
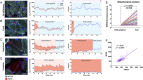
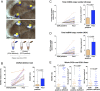
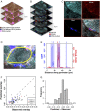
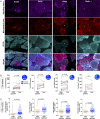
Results: We report respiratory chain-deficient perinuclear foci containing mtDNA deletions, which show local elevations of both mitochondrial mass and mtDNA copy number. These subcellular foci of respiratory chain deficiency are associated with a local increase in mitochondrial biogenesis and unfolded protein response signaling pathways. We also find that the commonly reported segmental pattern of mitochondrial deficiency is consistent with the three-dimensional organization of the human skeletal muscle mitochondrial network.
Interpretation: We propose that mtDNA deletions first exceed the biochemical threshold causing biochemical deficiency in focal regions adjacent to the myonuclei, and induce mitochondrial biogenesis before spreading across the muscle fiber. These subcellular resolution data provide new insights into the possible origin of mitochondrial respiratory chain deficiency in mitochondrial myopathy. Ann Neurol 2018;84:289-301.
© 2018 The Authors. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures



References
-
- de Grey AD. A proposed refinement of the mitochondrial free radical theory of aging. Bioessays 1997;19:161–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/L016354/1/MRC_/Medical Research Council/United Kingdom
- MC_UP_A620_1014/MRC_/Medical Research Council/United Kingdom
- R35 GM119793/GM/NIGMS NIH HHS/United States
- 203105/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MR/K501074/1/MRC_/Medical Research Council/United Kingdom
- G0800674/MRC_/Medical Research Council/United Kingdom
- G0400491/MRC_/Medical Research Council/United Kingdom
- MC_U147585824/MRC_/Medical Research Council/United Kingdom
- MC_U147585827/MRC_/Medical Research Council/United Kingdom
- R35GM119793/NH/NIH HHS/United States
- MC_U147585819/MRC_/Medical Research Council/United Kingdom
- MR/K000608/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/1/MRC_/Medical Research Council/United Kingdom
- F-1401/PUK_/Parkinson's UK/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- DH_/Department of Health/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

