Susceptibility of Human Prion Protein to Conversion by Chronic Wasting Disease Prions
- PMID: 30014840
- PMCID: PMC6056132
- DOI: 10.3201/eid2408.161888
Susceptibility of Human Prion Protein to Conversion by Chronic Wasting Disease Prions
Abstract
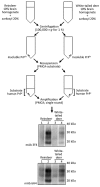
Chronic wasting disease (CWD) is a contagious and fatal neurodegenerative disease and a serious animal health issue for deer and elk in North America. The identification of the first cases of CWD among free-ranging reindeer and moose in Europe brings back into focus the unresolved issue of whether CWD can be zoonotic like bovine spongiform encephalopathy. We used a cell-free seeded protein misfolding assay to determine whether CWD prions from elk, white-tailed deer, and reindeer in North America can convert the human prion protein to the disease-associated form. We found that prions can convert, but the efficiency of conversion is affected by polymorphic variation in the cervid and human prion protein genes. In view of the similarity of reindeer, elk, and white-tailed deer in North America to reindeer, red deer, and roe deer, respectively, in Europe, a more comprehensive and thorough assessment of the zoonotic potential of CWD might be warranted.
Keywords: Europe; North America; chronic wasting disease; deer; elk; prion; protein misfolding cyclic amplification; reindeer; sheep; zoonoses.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

