A selective gut bacterial bile salt hydrolase alters host metabolism
- PMID: 30014852
- PMCID: PMC6078496
- DOI: 10.7554/eLife.37182
A selective gut bacterial bile salt hydrolase alters host metabolism
Abstract
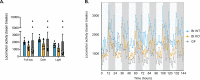
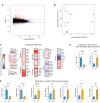
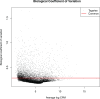
The human gut microbiota impacts host metabolism and has been implicated in the pathophysiology of obesity and metabolic syndromes. However, defining the roles of specific microbial activities and metabolites on host phenotypes has proven challenging due to the complexity of the microbiome-host ecosystem. Here, we identify strains from the abundant gut bacterial phylum Bacteroidetes that display selective bile salt hydrolase (BSH) activity. Using isogenic strains of wild-type and BSH-deleted Bacteroides thetaiotaomicron, we selectively modulated the levels of the bile acid tauro-β-muricholic acid in monocolonized gnotobiotic mice. B. thetaiotaomicron BSH mutant-colonized mice displayed altered metabolism, including reduced weight gain and respiratory exchange ratios, as well as transcriptional changes in metabolic, circadian rhythm, and immune pathways in the gut and liver. Our results demonstrate that metabolites generated by a single microbial gene and enzymatic activity can profoundly alter host metabolism and gene expression at local and organism-level scales.
Keywords: bacteroides thetaiotaomicron; bile salt hydrolase; biochemistry; chemical biology; human microbiome; infectious disease; metabolism; microbiology; mouse.
© 2018, Yao et al.
Conflict of interest statement
LY, SN, AA, ND, AM, AB, LB No competing interests declared, SS is currently affiliated with Indigo Agriculture, but the research was conducted when she was a Research Associate at Harvard Medical School. The author has no other competing interests to declare, AD is a consultant for Kintai Therapeutics
Figures





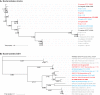
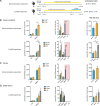
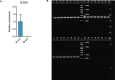

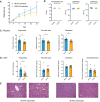

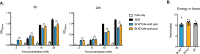

References
-
- Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Bäckman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. PNAS. 1998;95:12208–12213. doi: 10.1073/pnas.95.21.12208. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

