Neuropathic pain clinical trials: factors associated with decreases in estimated drug efficacy
- PMID: 30015707
- PMCID: PMC6193835
- DOI: 10.1097/j.pain.0000000000001340
Neuropathic pain clinical trials: factors associated with decreases in estimated drug efficacy
Abstract
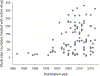
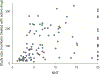
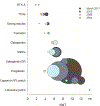
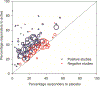
Multiple recent pharmacological clinical trials in neuropathic pain have failed to show beneficial effect of drugs with previously demonstrated efficacy, and estimates of drug efficacy seems to have decreased with accumulation of newer trials. However, this has not been systematically assessed. Here, we analyze time-dependent changes in estimated treatment effect size in pharmacological trials together with factors that may contribute to decreases in estimated effect size. This study is a secondary analysis of data from a previous published NeuPSIG systematic review and meta-analysis, updated to include studies published up till March 2017. We included double-blind, randomized, placebo-controlled trials examining the effect of drugs for which we had made strong or weak recommendations for use in neuropathic pain in the previously published review. As the primary outcome, we used an aggregated number needed to treat for 50% pain reduction (alternatively 30% pain reduction or moderate pain relief). Analyses involved 128 trials. Number needed to treat values increased from around 2 to 4 in trials published between 1982 and 1999 to much higher (less effective) values in studies published from 2010 onwards. Several factors that changed over time, such as larger study size, longer study duration, and more studies reporting 50% or 30% pain reduction, correlated with the decrease in estimated drug effect sizes. This suggests that issues related to the design, outcomes, and reporting have contributed to changes in the estimation of treatment effects. These factors are important to consider in design and interpretation of individual study data and in systematic reviews and meta-analyses.
Conflict of interest statement
Conflicts of interest statement
NA received speakers fee from Pfizer and reported consultant fees from Novartis, Teva, Grünenthal, Mundipharma, Sanofi Pasteur, Aptynix. RB received speakers or consultancy fees from Pfizer, Genzyme GmbH, Grünenthal GmbH, Mundipharma, Allergan, Sanofi Pasteur, Medtronic, Eisai, Lilly GmbH, Boehringer Ingelheim Pharma GmbH&Co.KG, Astellas, Novartis, Bristol-Myers Squibb, Biogenidec, AstraZeneca, Merck, Abbvie, Daiichi Sankyo, Glenmark Pharmaceuticals, Seqirus, Teva Pharma, Genentech, Galapagos NV, Kyowa Kirin GmbH, Vertex Pharmaceuticals Inc., Biotest AG, Celgene, Densitin, Bayer-Schering, MSD, TAD Pharma GmbH, and research support from Pfizer, Genzyme GmbH, Grünenthal GmbH, Mundipharma. RB is member of the EU Project No 633491: DOLORisk. Member of the IMI „Europain” collaboration and industry members of this are: Astra Zeneca, Pfizer, Esteve, UCB-Pharma, Sanofi Aventis, Grünen-thal GmbH, Eli Lilly and Boehringer Ingelheim Pharma GmbH&Co.KG, German Federal Ministry of Education and Research (BMBF), the ERA_NET NEURON / IM-PAIN Project (01EW1503), the German Research Network on Neuropathic Pain (01EM0903), NoPain system biology (0316177C) and the German Research Foundation (DFG). RHD has received in the past 36 months research grants and contracts from US Food and Drug Administration and US National Institutes of Health, and compensation from Abide, Adynxx, Aptinyx, Astellas, AstraZeneca, Biogen, Boston Scientific, Braeburn, Celgene, Centrexion, Chromocell, Concert, Coronado, Daiichi Sankyo, Dong-A, Eli Lilly, Eupraxia, Glenmark, Grace, Hope, Hydra, Immune, Johnson & Johnson, Medavante, Novartis, NSGene, Olatec, Periphagen, Pfizer, Phosphagenics, Quark, Reckitt Benckiser, Regenacy, Relmada, Sandoz, Semnur, Spinifex, Syntrix, Teva, Thar, Trevena, and Vertex. NBF has received honoraria for serving on advisory boards or speakers panels from Grünenthal, Teva Pharmaceuticals, Novartis Pharma, Astellas, and Mitshubishe Tanabe Pharma. IG has received support from Biogen, Adynxx, TARIS Biomedical, AstraZeneca, Pfizer, and Johnson and Johnson and has received grants from the Canadian Institutes of Health Research, Physicians’ Services Incorporated Foundation, and Queen’s University. SH has received a research grant from Pfizer Inc. MH received fees for consulting from Abbvies, Astellas, and Pfizer, and for lecturing from Astellas, Mundipharma, and Pfizer. TSJ received speakers or consultancy fees from Pfizer, Mundipharma, Daichi-Sankyo, Novartis, Orion Pharma and Biogen. PK has received payment for consultancy work or as a speaker for Janssen Pharmaceutica, Partners in Research, and Reckitt Benckiser. AM has received grant support from Grünenthal and Novartis and has received honoraria for attending boards with RB and honoraria from Omega Pharma and Futura Pharma. SNR has served on advisory boards for Allergan, Aptinyx, and Daichi-Sankyo. SNR is supported by an NIH grant- NS26363. ASCR has received funding from Orion Pharma, and undertakes consultancy and advisory board work for Imperial College Consultants in the last 12 months this has included remunerated work for: Merck, Galapagos, Toray, Quartet, Lateral, Novartis and Orion. ASCR was the owner of share options in Spinifex Pharmaceuticals from which personal benefit accrued upon the acquisition of Spinifex by Novartis in July 2015 and from which future milestone payments may occur. ASCR is named as an inventor on patents related to N-(2-propenyl) hexadecanamide and related amides and inhibition of vgf activity. NTA, BHS, EM, and ES have no conflicts of interests.
Figures








References
-
- Dworkin RH, Turk DC, Peirce-Sandner S, Burke LB, Farrar JT, Gilron I, Jensen MP, Katz NP, Raja SN, Rappaport BA, Rowbotham MC, Backonja MM, Baron R, Bellamy N, Bhagwagar Z, Costello A, Cowan P, Fang WC, Hertz S, Jay GW, Junor R, Kerns RD, Kerwin R, Kopecky EA, Lissin D, Malamut R, Markman JD, McDermott MP, Munera C, Porter L, Rauschkolb C, Rice AS, Sampaio C, Skljarevski V, Sommerville K, Stacey BR, Steigerwald I, Tobias J, Trentacosti AM, Wasan AD, Wells GA, Williams J, Witter J, Ziegler D. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain 2012;153:1148–1158. - PubMed
-
- Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 2005;118:289–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

