Effect of celastrol on toll‑like receptor 4‑mediated inflammatory response in free fatty acid‑induced HepG2 cells
- PMID: 30015859
- PMCID: PMC6108865
- DOI: 10.3892/ijmm.2018.3775
Effect of celastrol on toll‑like receptor 4‑mediated inflammatory response in free fatty acid‑induced HepG2 cells
Abstract
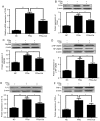
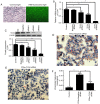
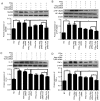
Toll‑like receptor 4 (TLR4)‑mediated immune and inflammatory signaling serves a pivotal role in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Our previous study demonstrated that celastrol treatment was able to improve hepatic steatosis and inhibit the TLR4 signaling cascade pathway in type 2 diabetic rats. The present study aimed to investigate the effects of celastrol on triglyceride accumulation and inflammation in steatotic HepG2 cells, and the possible mechanisms responsible for the regulation of cellular responses following TLR4 gene knockdown by small interfering RNA (siRNA) in vitro. A cell model of hepatic steatosis was prepared by exposing the HepG2 cells to free fatty acid (FFA) in the absence or presence of celastrol. Intracellular triglycerides were visualized by Oil red O staining, and the TLR4/myeloid differentiation primary response 88 (MyD88)/nuclear factor‑κB (NF‑κB) signaling cascade pathway were investigated. To directly elucidate whether TLR4 was the blocking target of celastrol upon FFA exposure, the cellular response to inflammation was determined upon transfection with TLR4 siRNA. The results revealed that celastrol significantly reduced triglyceride accumulation in the steatotic HepG2 cells, and downregulated the expression levels of TLR4, MyD88 and phospho‑NF‑κBp65, as well as of the downstream inflammatory cytokines interleukin‑1β and tumor necrosis factor α. Knockdown of TLR4 also alleviated FFA‑induced inflammatory response. In addition, co‑treatment with TLR4 siRNA and celastrol further attenuated the expression of inflammatory mediators. These results suggest that celastrol exerts its protective effect partly via inhibiting the TLR4‑mediated immune and inflammatory response in steatotic HepG2 cells.
Figures
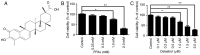

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

