Improving rational use of ACTs through diagnosis-dependent subsidies: Evidence from a cluster-randomized controlled trial in western Kenya
- PMID: 30016316
- PMCID: PMC6049880
- DOI: 10.1371/journal.pmed.1002607
Improving rational use of ACTs through diagnosis-dependent subsidies: Evidence from a cluster-randomized controlled trial in western Kenya
Abstract
Background: More than half of artemisinin combination therapies (ACTs) consumed globally are dispensed in the retail sector, where diagnostic testing is uncommon, leading to overconsumption and poor targeting. In many malaria-endemic countries, ACTs sold over the counter are available at heavily subsidized prices, further contributing to their misuse. Inappropriate use of ACTs can have serious implications for the spread of drug resistance and leads to poor outcomes for nonmalaria patients treated with incorrect drugs. We evaluated the public health impact of an innovative strategy that targets ACT subsidies to confirmed malaria cases by coupling free diagnostic testing with a diagnosis-dependent ACT subsidy.
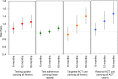
Methods and findings: We conducted a cluster-randomized controlled trial in 32 community clusters in western Kenya (population approximately 160,000). Eligible clusters had retail outlets selling ACTs and existing community health worker (CHW) programs and were randomly assigned 1:1 to control and intervention arms. In intervention areas, CHWs were available in their villages to perform malaria rapid diagnostic tests (RDTs) on demand for any individual >1 year of age experiencing a malaria-like illness. Malaria RDT-positive individuals received a voucher for a discount on a quality-assured ACT, redeemable at a participating retail medicine outlet. In control areas, CHWs offered a standard package of health education, prevention, and referral services. We conducted 4 population-based surveys-at baseline, 6 months, 12 months, and 18 months-of a random sample of households with fever in the last 4 weeks to evaluate predefined, individual-level outcomes. The primary outcome was uptake of malaria diagnostic testing at 12 months. The main secondary outcome was rational ACT use, defined as the proportion of ACTs used by test-positive individuals. Analyses followed the intention-to-treat principle using generalized estimating equations (GEEs) to account for clustering with prespecified adjustment for gender, age, education, and wealth. All descriptive statistics and regressions were weighted to account for sampling design. Between July 2015 and May 2017, 32,404 participants were tested for malaria, and 10,870 vouchers were issued. A total of 7,416 randomly selected participants with recent fever from all 32 clusters were surveyed. The majority of recent fevers were in children under 18 years (62.9%, n = 4,653). The gender of enrolled participants was balanced in children (49.8%, n = 2,318 boys versus 50.2%, n = 2,335 girls), but more adult women were enrolled than men (78.0%, n = 2,139 versus 22.0%, n = 604). At baseline, 67.6% (n = 1,362) of participants took an ACT for their illness, and 40.3% (n = 810) of all participants took an ACT purchased from a retail outlet. At 12 months, 50.5% (n = 454) in the intervention arm and 43.4% (n = 389) in the control arm had a malaria diagnostic test for their recent fever (adjusted risk difference [RD] = 9 percentage points [pp]; 95% CI 2-15 pp; p = 0.015; adjusted risk ratio [RR] = 1.20; 95% CI 1.05-1.38; p = 0.015). By 18 months, the ARR had increased to 1.25 (95% CI 1.09-1.44; p = 0.005). Rational use of ACTs in the intervention area increased from 41.7% (n = 279) at baseline to 59.6% (n = 403) and was 40% higher in the intervention arm at 18 months (ARR 1.40; 95% CI 1.19-1.64; p < 0.001). While intervention effects increased between 12 and 18 months, we were not able to estimate longer-term impact of the intervention and could not independently evaluate the effects of the free testing and the voucher on uptake of testing.
Conclusions: Diagnosis-dependent ACT subsidies and community-based interventions that include the private sector can have an important impact on diagnostic testing and population-wide rational use of ACTs. Targeting of the ACT subsidy itself to those with a positive malaria diagnostic test may also improve sustainability and reduce the cost of retail-sector ACT subsidies.
Trial registration: ClinicalTrials.gov NCT02461628.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- World Health Organization. World Malaria Report 2015. Geneva: 2015.
-
- Arrow KJ, Panosian C, Gelband H. Saving Lives, Buying Time: Economics of Malaria Drugs in an Age of Resistance Institute of Medicine Committee on the Economics of Antimalarial Drugs. Washington (DC): National Academies Press (US); 2004. - PubMed
-
- Tougher S, Ye Y, Amuasi JH, Kourgueni IA, Thomson R, Goodman C, et al. Effect of the Affordable Medicines Facility—malaria (AMFm) on the availability, price, and market share of quality-assured artemisinin-based combination therapies in seven countries: a before-and-after analysis of outlet survey data. Lancet. 2012;380:1916–26. 10.1016/S0140-6736(12)61732-2 - DOI - PubMed
-
- Tougher S, Hanson K, Goodman C. What happened to anti-malarial markets after the Affordable Medicines Facility-malaria pilot? Trends in ACT availability, price and market share from five African countries under continuation of the private sector co-payment mechanism. Malar J. 2017;16(1):173 Epub 2017/04/27. 10.1186/s12936-017-1814-z ; PubMed Central PMCID: PMCPmc5405529. - DOI - PMC - PubMed
-
- UNITAID. GLOBAL MALARIA DIAGNOSTIC AND ARTEMISININ TREATMENT COMMODITIES DEMAND FORECAST; 2017–2020. 2017.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

