Remote monitoring and clinical outcomes: details on information flow and workflow in the IN-TIME study
- PMID: 30016396
- PMCID: PMC6440440
- DOI: 10.1093/ehjqcco/qcy031
Remote monitoring and clinical outcomes: details on information flow and workflow in the IN-TIME study
Abstract
Aims: Randomized clinical trials investigating a possible outcome effect of remote monitoring in patients with implantable defibrillators have shown conflicting results. This study analyses the information flow and workflow details from the IN-TIME study and discusses whether differences of message content, information speed and completeness, and workflow may contribute to the heterogeneous results.
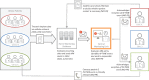
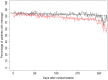
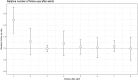
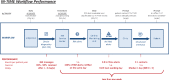
Methods and results: IN-TIME randomized 664 patients with an implantable cardioverter/defibrillator indication to daily remote monitoring vs. control. After 12 months, a composite clinical score and all-cause mortality were improved in the remote monitoring arm. Messages were received on 83.1% of out-of-hospital days. Daily transmissions were interrupted 2.3 times per patient-year for more than 3 days. During 1 year, absolute transmission success declined by 3.3%. Information on medical events was available after 1 day (3 days) in 83.1% (94.3%) of the cases. On all working days, a central monitoring unit informed investigators of protocol defined events. Investigators contacted patients with a median delay of 1 day and arranged follow-ups, the majority of which took place within 1 week of the event being available.
Conclusion: Only limited data on the information flow and workflow have been published from other studies which failed to improve outcome. However, a comparison of those data to IN-TIME suggest that the ability to see a patient early after clinical events may be inferior to the set-up in IN-TIME. These differences may be responsible for the heterogeneity found in clinical effectiveness of remote monitoring concepts.
Keywords: Defibrillator; Heart failure; Telemedicine; implantatble.
© The Author(s) 2018. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
References
-
- Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, Hayes DL, Howlett JG, Kautzner J, Love CJ, Morgan JM, Priori SG, Reynolds DW, Schoenfeld MH, Vardas PE.. HRS/EHRA Expert Consensus on the Monitoring of Cardiovascular Implantable Electronic Devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations: developed in partnership with the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA); and in collaboration with the American College of Cardiology (ACC), the American Heart Association (AHA), the European Society of Cardiology (ESC), the Heart Failure Association of ESC (HFA), and the Heart Failure Society of America (HFSA). Endorsed by the Heart Rhythm Society, the European Heart Rhythm Association (a registered branch of the ESC), the American College of Cardiology, the American Heart Association. Europace 2008;10:707–725. - PubMed
-
- Slotwiner D, Varma N, Akar JG, Annas G, Beardsall M, Fogel RI, Galizio NO, Glotzer TV, Leahy RA, Love CJ, McLean RC, Mittal S, Morichelli L, Patton KK, Raitt MH, Pietro Ricci R, Rickard J, Schoenfeld MH, Serwer GA, Shea J, Varosy P, Verma A, Yu C-M.. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm 2015;12:e69–e100. - PubMed
-
- Varma N, Epstein AE, Irimpen A, Schweikert R, Love C.. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010;122:325–332. - PubMed
-
- Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, Meyer TE, Jones PW, Boehmer JP.. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation 2010;122:2359–2367. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

